Details of the Drug Combinations
General Information of This Drug (ID: DM5YF4M)
| Drug Name | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
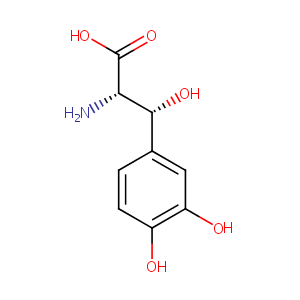
droxidopa; 23651-95-8; L-DOPS; Northera; (2S,3R)-2-amino-3-(3,4-dihydroxyphenyl)-3-hydroxypropanoic acid; threo-Dopaserine; 3916-18-5; DOPS; DL-threo-DOPS; L-Threodops; DL-threo-3,4-Dihydroxyphenylserine; DL-threo-Droxidopa; DL-threo-Dihydroxyphenylserine; UNII-J7A92W69L7; SM 5688; CHEBI:31524; Droxidopa (L-DOPS); threo-beta,3-Dihydroxy-DL-tyrosine; EINECS 223-480-5; beta,3-Dihydroxy-DL-tyrosine threo-; BRN 2852792; L-threo-dihydroxyphenylserine; DL-threo-3-(3,4-Dihydroxyphenyl)serine; Serine, 3-(3,4-dihydroxyphenyl)-, DL-threo-
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||
References
