Details of the Drug Combinations
General Information of This Drug (ID: DM7MDBE)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Vericiguat; 1350653-20-1; UNII-LV66ADM269; BAY-1021189; Methyl (4,6-diamino-2-(5-fluoro-1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl)pyrimidin-5-yl)carbamate; BAY1021189; LV66ADM269; MK-1242; Methyl {4,6-diamino-2-[5-fluoro-1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-5-yl}carbamate; methyl N-[4,6-diamino-2-[5-fluoro-1-[(2-fluorophenyl)methyl]pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-5-yl]carbamate; Methyl (4,6-diamino-2-(5-fluoro-1-(2-fluorobenzyl)-1H-pyrazolo(3,4-b)pyridin-3-yl)pyrimidin-5-yl)carbam
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
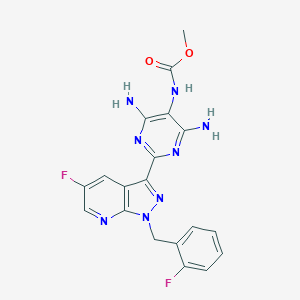 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||
References
