| 1 |
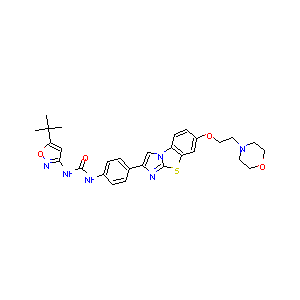
FDA Approved Drug Products from FDA Official Website. 2023. Application Number: 216993
|
| 2 |
ClinicalTrials.gov (NCT02039726) An Open-label Study of Quizartinib Monotherapy vs. Salvage Chemotherapy in Acute Myeloid Leukemia (AML) Subjects. U.S. National Institutes of Health.
|
| 3 |
ClinicalTrials.gov (NCT02834390) Study of Quizartinib in Japanese Patients With Newly Diagnosed Acute Myeloid Leukemia (AML)
|
| 4 |
ClinicalTrials.gov (NCT01411267) AC220 for Children With Relapsed/Refractory ALL or AML
|
| 5 |
ClinicalTrials.gov (NCT01892371) Quizartinib With Azacitidine or Cytarabine in Treating Patients With Relapsed or Refractory Acute Myeloid Leukemia or Myelodysplastic Syndrome
|
| 6 |
ClinicalTrials.gov (NCT04687761) Clinical Trial to Assess the Safety and Tolerability of the Combination of Low-dose Cytarabine or Azacitidine Plus Venetoclax and Quizartinib in Newly Diagnosed AML Patients Aged Equal or More Than 60 Years Old
|
| 7 |
ClinicalTrials.gov (NCT03735875) Venetoclax and Quizartinib in Treating Patients With FLT3-mutated Recurrent or Refractory Acute Myeloid Leukemia
|
| 8 |
ClinicalTrials.gov (NCT04107727) Trial to Compare Efficacy and Safety of Chemotherapy/Quizartinib vs Chemotherapy/Placebo in Adults FMS-like Tyrosine Kinase 3 (FLT3) Wild-type Acute Myeloid Leukemia (AML)
|
| 9 |
ClinicalTrials.gov (NCT02668653) Quizartinib With Standard of Care Chemotherapy and as Continuation Therapy in Patients With Newly Diagnosed FLT3-ITD (+) Acute Myeloid Leukemia (AML)
|
|
|
|
|
|
|