Details of the Drug Combinations
General Information of This Drug (ID: DMC6U93)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Torin 2; 1223001-51-1; Torin-2; 9-(6-aminopyridin-3-yl)-1-[3-(trifluoromethyl)phenyl]benzo[h][1,6]naphthyridin-2-one; 9-(6-AMINO-3-PYRIDINYL)-1-[3-(TRIFLUOROMETHYL)PHENYL]-BENZO[H]-1,6-NAPHTHYRIDIN-2(1H)-ONE; CHEMBL1765602; C24H15F3N4O; CHEBI:90682; 9-(6-Aminopyridin-3-Yl)-1-[3-(Trifluoromethyl)phenyl]benzo[h][1,6]naphthyridin-2(1h)-One; 9-(6-AMINOPYRIDIN-3-YL)-1-(3-(TRIFLUOROMETHYL)PHENYL)BENZO[H][1,6]NAPHTHYRIDIN-2(1H)-ONE; 9-(6-Aminopyridin-3-yl)-1-(3-(trifluoromethyl)-phenyl)benzo[h][1,6]naphthyridin-2(1H)-one; BENZO[H]-1,6-NAPHTHYRIDIN-2(1H)-ONE, 9-(6-AMINO-3-PYRIDINYL)-1-[3-(TRIFLUOROMETHYL)PHENYL]-; 9-(6-Amino-3-pyridinyl)-1-[3-(trifluoromethyl)phenyl]benzo[h]-1,6-naphthyridin-2(1H)-one; cc-275; MLS006011167; GTPL8839; SCHEMBL6876328; AOB3537; DTXSID00679917; EX-A431; HMS3265O05; HMS3265O06; HMS3265P05; HMS3265P06; HMS3651N13; BCP02612; ABP000908; BDBM50341209; MFCD18782652; NSC775727; s2817; ZINC71318831; AKOS024458055; CCG-265003; CS-0236; NSC-775727; PB34957; NCGC00263216-01; NCGC00263216-02; NCGC00263216-09; NCGC00263216-13; 9-(6-AMINO-PYRIDIN-3-YL)-1-(3-TRIFLUOROMETHYL-PHENYL)-1H-BENZO[H][1,6]NAPHTHYRIDIN-2-ONE; AC-31520; AK171126; AS-74405; HY-13002; SMR004702936; AB0035864; DB-084736; FT-0700124; SW218309-2; Y0293; Q-4148; J-519481; BRD-K68174511-001-01-7; Q27089008; 9-(6-amino-3-pyridyl)-1-[3-(trifluoromethyl)phenyl]benzo[h][1,6]naphthyridin-2-one; 17G; 9-(6-Amino-3-pyridinyl)-1-[3-(trifl uoromethyl)phenyl]-benzo[h]-1,6-naphthyridin-2(1H) -one; 9-(6-AMINOPYRIDIN-3-YL)-1-[3-(TRIFLUOROMETHYL)PHENYL]-1H,2H-BENZO[H]1,6-NAPHTHYRIDIN-2-ONE
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
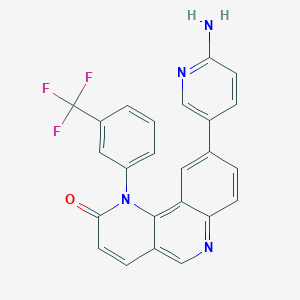 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
5 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||||||||||||||||||||||
References
