Details of the Drug Combinations
General Information of This Drug (ID: DMGEMB7)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Acticort; Alacort; Algicirtis; Alphaderm; Amberin; Anflam; Anucort; Aquacort; Cetacort; Chronocort; Cleiton; Cobadex; Colocort; Corhydron; Cortanal; Cortef; Cortenema; Cortesal; Corticreme; Cortifair; Cortifan; Cortiment; Cortisol; Cortisolonum; Cortisporin; Cortispray; Cortolotion; Cortonema; Cortoxide; Cortril; Cremesone; Cutisol; Delacort; Dermacort; Dermaspray; Dermil; Dermocortal; Dermolate; Dihydrocostisone; Dioderm; Drotic; DuoCort; Efcorbin; Efcortelan; Efcortelin; Eldecort; Eldercort; Epicort; Epicortisol; Evacort; Ficortril; Fiocortril; Flexicort; Genacort; Glycort; Hidalone; Hycort; Hycortol; Hycortole; Hydracort; Hydrasson; Hydrocortal; Hydrocorticosterone; Hydrocortisyl; Hydrocortone; Hydroskin; Hydroxycortisone; Hytisone; Hytone; Idrocortisone; Kyypakkaus; Lactisona; Lubricort; Maintasone; Medicort; Meusicort; Mildison; Milliderm; Nutracort; Optef; Otocort; Penecort; Permicort; Prepcort; Proctocort; Protocort; Rectoid; Sanatison; Schericur; Sigmacort; Signef; Stiefcorcil; Synacort; Tarcortin; Texacort; Timocort; Traumaide; Uniderm; Vytone; Zenoxone; ACETASOL HC; Aeroseb HC; AnusolHC; Aquanil HC; Barseb HC; CaldeCORT Spray; Clear aid; Component of Lubricort; Component of Otalgine; Cortisol alcohol; Cortisporin Otico; Ef corlin; Epiderm H; Esiderm H; Foille Insetti; HYDROCORTISONE AND ACETIC ACID; HYDROCORTISONE IN ABSORBASE; Heb Cort; Hydrocortisone alcohol; Hydrocortisone base; Hydrocortisone free alcohol; Hydrocortisone solution; Hytone lotion; Idrocortisone [DCIT]; Komed HC; Lacticare HC; Nogenic HC; ORLEX HC; Pediotic Suspension; Polcort H; Preparation H Hydrocortisone Cream; Prevex HC; Proctozone HC; Remederm HC; Scalpicin Capilar; Scheroson F; Systral Hydrocort; Transderma H; VoSol HC; H 4001; Texacort lotion 25; [3H]cortisol; ACETIC ACID W/ HYDROCORTISONE; Acticort (TN); Aeroseb-HC; Ala-Cort; Ala-Scalp; Anti-inflammatory hormone; Anucort-HC; Anusol HC (TN); Balneol-hc; Basan-Corti; Beta-hc; COR-OTICIN; Colocort (TN); Cort-Dome; Cortef (TN); Cortisol, Hydrocortisone; Cremicort-H; Cyclodextrin-encapsulated hydrocortisone; Derm-Aid; Dome-cort; Domolene-HC; Genacort (lotion); Gyno-Cortisone; H-Cort; HC #1; HC #4; HC (HYDROCORTISONE); Heb-Cort; Hi-cor; Hidro-Colisona; Hidrocortisona [INN-Spanish]; Hydro-Adreson; Hydro-Colisona; Hydro-RX; Hydrocortisone-Water Soluble; Hydrocortisonum [INN-Latin]; Hytone (TN); Incortin-H; Incortin-hydrogen; Kendall's compound F; Lacticare-HC; Neo-Cortef; Neosporin-H Ear; Nystaform-HC; Otosone-F; Rectasol-HC; Reichstein's substance M; Scalp-Cort; Stie-cort; Component of Neo-Cort-Dome; Hydrocortisone [INN:BAN:JAN]; Hydrocortisone (JP15/USP/INN); 11-beta-Hydrocortisone; 11-beta-Hydroxycortisone; 11beta,17,21-Trihydroxyprogesterone; 11beta-Hydrocortisone; 11beta-Hydroxycortisone; 17-Hydroxycorticosterone; 17alpha-Hydroxycorticosterone
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiinflammatory Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
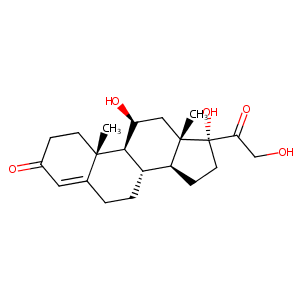 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
14 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
