Details of the Drug Combinations
General Information of This Drug (ID: DMI347A)
| Drug Name | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
lamivudine; 134678-17-4; Epivir; Zeffix; Heptovir; Epivir-HBV; Hepitec; Heptodin; BCH-189; 3TC; Heptivir; CIS-LAMIVUDINE; (-)-2'-Deoxy-3'-thiacytidine; 4-amino-1-((2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)pyrimidin-2(1H)-one; GR-109714X; 3'-Thia-2',3'-dideoxycytidine; (-)-BCH-189; Lamivudine [USAN:BAN:INN]; GR109714X; beta-L-3'-Thia-2',3'-dideoxycytidine; beta-L-2',3'-Dideoxy-3'-thiacytidine; (-)NGPB-21; 136891-12-8; 2',3'-Dideoxy-3'-thiacytidine; (-)-BCH 189; UNII-2T8Q726O95; HSDB 7155; GR 109714X; DTHC; LMV; Lamivir; Zefix; BCH 189; BCH189; BCH-790; DRG-0126; Epivir (TN); Epivir(TM); GG-714; HHA & 3TC; HHA & Lamivudine; Heptovir (TN); Lamivudine & GNA; Zeffix (TN); Epivir-HBV (TN); Lamivudine [USAN:INN:BAN]; Lamivudine (JAN/USP/INN); Lamivudine, (2S-cis)-Isomer; Beta-L-2',3'-Dideoxy-3'-thiacytidine; Beta-L-3'-Thia-2',3'-dideoxycytidine; Beta-L-(-)-2',3'-dideoxy-3'-thiacytidine & Sho-Saiko-To; (+/-)-(Cis)-1-[2-(Hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine; (+/-)-3TC; (+/-)-BCH-189; (+/-)-SddC; (-)-(2'R,5'S)-1-[2'-Hydroxymethyl-5'-(1,3-oxathiolanyl)]cytosine; (-)-1-((2R,5S)-2-(Hydroxymethyl)-1,3-oxathiolan-5-yl)cytosine; (-)-1-[(2R,5S)-2-(Hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine; (-)-NGPB-21; (-)-SddC; (-)-beta-L-2',3'-Dideoxy-3'-thiacytidine; (2R,cis)-4-amino-1-(2-hydroxymethyl-1,3-oxathiolan-5-yl)-(1H)-pyrimidin-2-one; 2',3' Dideoxy 3' thiacytidine; 2(1H)-Pyrimidinone, 4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl], (+/-)-(Cis); 2(1H)-Pyrimidinone, 4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl], (-)-(2R,5S); 2(1H)-Pyrimidinone, 4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl], (-)-(2R,5S) & Galanthus Nivalis Agglutinin (GNA); 2(1H)-Pyrimidinone, 4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl], (-)-(2R,5S) & Hippeastrum hybrid agglutinin(HHA); 3TC & GNA; 3TC & SST; 3TC (AIDS INITIATIVE) (AIDS INITIATIVE); 3TC and NV-01; 3TC, Zeffix, Heptovir, Epivir, Epivir-HBV, Lamivudine; 4-Amino-1-((2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)-2(1H)-pyrimidinone; 4-amino-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2(1H)-one; 4-amino-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2-one; Efavirenz/lamivudine/tenofovir fumarate
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Anti-HIV Agents
|
||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
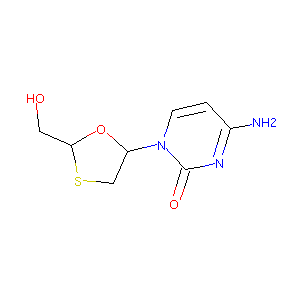 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
3 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
34 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
