Details of the Drug Combinations
General Information of This Drug (ID: DMIXC7G)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Aclasta; Reclast; ZOL; Zometa; Novartis brand of zoledronic acid; Zoledronic acid; Zometa Concentrate; Bisphosphonate 3; CGP 42446; CGP 42446A; Aclasta (TN); CGP 42'446; CGP-42446; KS-1132; Reclast (TN); Zoledronic acid (INN); Zoledronic acid [USAN:INN]; Zomera (TN); Zometa (Novartis); Zometa (TN); CGP-42'446; Zometa, Zomera, Aclasta and Reclast, Zoledronic Acid; [1-hydroxy-2-(1H-imidazol-1-yl)ethane-1,1-diyl]bis(phosphonic acid); (1-Hydroxy-2-imidazol-1-ylethylidene)diphosphonic acid; (1-hydroxy-2-(1H-imidazol-1-yl)ethylidene)bisphosphonic acid; (1-hydroxy-2-imidazol-1-yl-1-phosphonoethyl)phosphonic acid; (1-hydroxy-2-imidazol-1-yl-phosphonoethyl)phosphonic acid monohydrate; 2-(imidazol-1-yl)-1-hydroxyethane-1,1-diphosphonic acid; 2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosphonic acid
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Bone Density Conservation Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
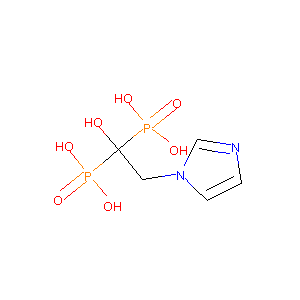 |
|||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
13 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
14 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
