Details of the Drug Combinations
General Information of This Drug (ID: DMOBIKY)
| Drug Name | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Bexaroteno; Bexarotenum; Targret; Targretin; Targretyn; Targrexin; Bexarotene [USAN]; Elan brand of bexarotene;Ligand brand of bexarotene; LG 1069; LG100069; LG1069; LG69 compound; LGD 1069; LGD1069; LG-100069; LGD-1069; Targretin (TN); Targretin-gel; Bexarotene (USAN/INN); P-(1-(5,6,7,8-Tetrahydro-3,5,5,8,8-pentamethyl-2-naphthyl)vinyl)benzoic acid; 3-methyl-TTNEB; 4-(1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthyl)ethenyl)benzoic acid; 4-(1-(5,6,7,8-Tetrahydro-3,5,5,8,8-pentamethyl-2-naphthalenyl)ethenyl)benzoic acid; 4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaphthalen-2-yl)ethenyl]benzoic acid; 4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaphthalen-2-yl)vinyl]benzoic acid; 4-[1-(3,5,5,8,8-pentamethyl-6,7-dihydronaphthalen-2-yl)ethenyl]benzoic acid; 4-[1-3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthaleneyl)vinyl]benzene carboxylic acid; 9RA
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
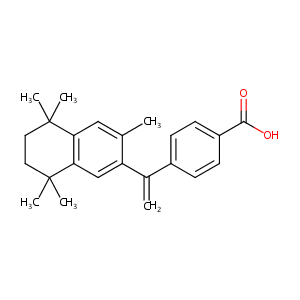 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
6 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
