Details of the Drug Combinations
General Information of This Drug (ID: DMSCDY9)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Diosmol; Hexahydroxyhexane; Invenex; Isotol; MTL; Manita; Mannazucker; Mannidex; Mannigen; Mannistol; Mannit; Mannite; Osmitrol; Osmofundin; Osmosal; Resectisol; Cordycepic acid; Cpd without stereochemical designation; Manna sugar; Mannitol [USAN]; Marine Crystal; Resectisol In Plastic Container; M0044; Mannidex 16700; Mannogem 2080; D-mannite; D-mannitol; DL-Mannitol; MANNITOL 25%; Maniton-S; Mannitol (USP); Mannitol (VAN); Mannitol 10%; Mannitol 10% In Plastic Container; Mannitol 15%; Mannitol 15% In Plastic Container; Mannitol 20%; Mannitol 20% In Plastic Container; Mannitol 5%; Mannitol 5% In Plastic Container; OSMITROL 10% IN WATER IN PLASTIC CONTAINER; OSMITROL 15% IN WATER IN PLASTIC CONTAINER; OSMITROL 20% IN WATER IN PLASTIC CONTAINER; OSMITROL 5% IN WATER IN PLASTIC CONTAINER; Osmitrol (TN); Osmitrol 10% In Water; Osmitrol 15% In Water; Osmitrol 20% In Water; Osmitrol 5% In Water; SDM No. 35; SORBITOL-MANNITOL IN PLASTIC CONTAINER; Sorbitol-Mannitol; D-Mannitol (JP15); MANNITOL 10% W/ DEXTROSE 5% IN DISTILLED WATER; D-(-)-Mannitol; ED1D1E61-FEFB-430A-AFDC-D1F4A957FC3D; MANNITOL 15% DEXTROSE 5% IN SODIUM CHLORIDE 0.45%; MANNITOL 5% DEXTROSE 5% IN SODIUM CHLORIDE 0.12%; (2R,3R,4R,5R)-hexane-1,2,3,4,5,6-hexol; (L)-Mannitol
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Sweetening Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
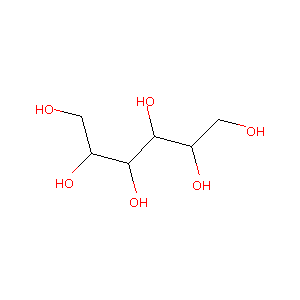 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
4 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||
References
