Details of the Drug
General Information of Drug (ID: DM429AE)
| Drug Name |
Levomethadyl acetate hydrochloride
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Levacetilmetadol; Levacetilmetadol [INN-Spanish]; Levacetylmethadol; Levacetylmethadol (INN); Levacetylmethadol [INN]; Levacetylmethadolum; Levacetylmethadolum [INN-Latin]; Levomethadyl; Levomethadyl acetate; Levacetyl methadol hydrochloride; LAAM hydrochloride; Levomethadyl acetate HCl; MK 790; MK-790 hydrochloride; ORLAAM hydrochloride; l-Acetylmethadol hydrochloride; (-)-(3S,6S)-6-(Dimethylamino)-4,4-diphenyl-3-heptanol acetate (ester), hydrochloride; 43033-72-3; 6-Dimethylamino-4,4-diphenyl-3-heptanolacetate.HCl; B54CW5KG52; CCRIS 3321; Levomethadyl acetate hydrochloride; Levomethadyl acetate hydrochloride [USAN]; UNII-B54CW5KG52; alpha-l-Acetylmethadol hydrochloride; l-alpha-Acetylmethadol hydrochloride; N-alpha-Acetylmethadol; Orlaam; alpha-l-Acetylmethadol; l-alpha-Acetylmethadol; levo-Alphacetylmethadol; levo-Methadyl acetate; levo-alpha-Acetylmethadol; (-)-alpha-Acetylmethadol; 1-alpha-Acetylmethadol; 1477-40-3; DEA No. 9648; LAAM; UNII-R3B637Y991; alpha-(-)-Acetylmethadol
|
|||||
| Structure |
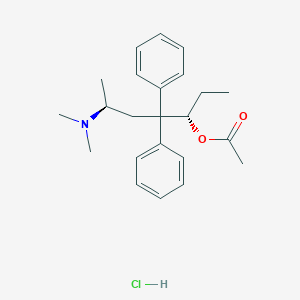 |
|||||
| 3D MOL is unavailable | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 390 | ||||
| Logarithm of the Partition Coefficient | Not Available | |||||
| Rotatable Bond Count | 9 | |||||
| Hydrogen Bond Donor Count | 1 | |||||
| Hydrogen Bond Acceptor Count | 3 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
References
