Details of the Drug
General Information of Drug (ID: DMHO29Y)
| Drug Name |
T83193
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
vanillin; 4-Hydroxy-3-methoxybenzaldehyde; 121-33-5; Vanillaldehyde; Vanillic aldehyde; Vanilla; p-Vanillin; 2-Methoxy-4-formylphenol; Vanilline; Lioxin; 4-Hydroxy-m-anisaldehyde; 3-Methoxy-4-hydroxybenzaldehyde; Zimco; Benzaldehyde, 4-hydroxy-3-methoxy-; 4-Hydroxy-3-methoxy-benzaldehyde; Vanilin; p-Hydroxy-m-methoxybenzaldehyde; 4-Formyl-2-methoxyphenol; Methylprotocatechuic aldehyde; Vanillin (natural); 4-Hydroxy-5-methoxybenzaldehyde; m-Anisaldehyde, 4-hydroxy-; Protocatechualdehyde, methyl-; Vanillin [USAN]; Vanillin (NF)
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
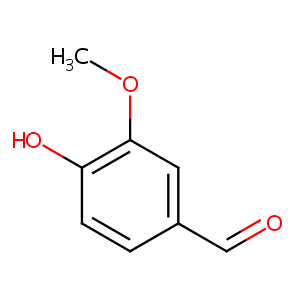 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 152.15 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
