Details of the Drug
General Information of Drug (ID: DMLSUWZ)
| Drug Name |
PSI-7977
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1190307-88-0; SOVALDI; PSI 7977; GS-7977; GS 7977; UNII-WJ6CA3ZU8B; WJ6CA3ZU8B; CHEBI:85083; Sofosbuvir (PSI-7977, GS-7977); Hepcvir; Hepcinat; HSDB 8226; 2-((5-(2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl)-4-fluoro-3-hydroxy-4-methyltetrahydrofuran-2-ylmethoxy)phenoxyphosphorylamino)propionic acid isopropyl ester; Sofosbuvir [USAN:INN]; Resof; SoviHep; Sovaldi (TN); Sofosbuvir (JAN/USAN); Sofosbuvir(PSI-7977); GTPL7368; SCHEMBL2010114; CHEMBL1259059; AMMD00019; EX-A389; MolPort-028-720-482; isopropyl (2S)-2-[[[(2R,
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Affected Organisms |
Hepatitis C Virus
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
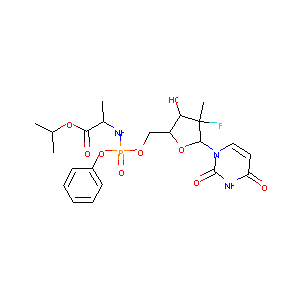 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 3 | Molecular Weight (mw) | 529.5 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 11 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 11 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from PSI-7977 (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | 2016 FDA drug approvals. Nat Rev Drug Discov. 2017 Feb 2;16(2):73-76. | ||||
|---|---|---|---|---|---|
| 2 | 2011 Pipeline of Pharmasset. | ||||
| 3 | Radium 223 dichloride for prostate cancer treatment. Drug Des Devel Ther. 2017 Sep 6;11:2643-2651. | ||||
| 4 | Mechanism of activation of PSI-7851 and its diastereoisomer PSI-7977. J Biol Chem. 2010 Nov 5;285(45):34337-47. | ||||
| 5 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 6 | Product Information. Balversa (erdafitinib). Janssen Products, LP, Horsham, PA. | ||||
| 7 | Product Information. Tukysa (tucatinib). Seattle Genetics Inc, Bothell, WA. | ||||
| 8 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 9 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
