Details of the Drug
General Information of Drug (ID: DMQ45WH)
| Drug Name |
3,5-dichlorosalicylic acid
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
3,5-DICHLOROSALICYLIC ACID; 3,5-Dichloro-2-hydroxybenzoic acid; 320-72-9; Benzoic acid, 3,5-dichloro-2-hydroxy-; Salicylic acid, 3,5-dichloro-; USAF DO-68; 2-Hydroxy-3,5-dichlorobenzoic acid; 3,5-Dichlorosalicylicacid; UNII-O6PXR32G3V; 3,5-Dichlorosalicyclic acid; HSDB 5562; EINECS 206-281-8; NSC 30109; 3,5-dichloro-2-hydroxy-benzoic acid; BRN 2210803; O6PXR32G3V; AI3-22601; CHEMBL449129; CNJGWCQEGROXEE-UHFFFAOYSA-N; C2U; ACMC-1CUK7; Salicylic acid,5-dichloro-; AC1Q3M9M; WLN: QVR BQ CG EG; DSSTox_RID_80582; DSSTox_CID_24914
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
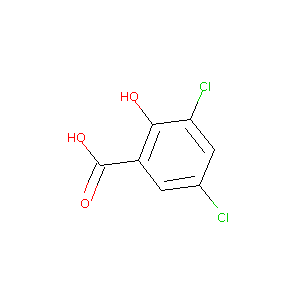 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 207.01 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
