| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 3 |
2004 approvals: the demise of the blockbuster. Nat Rev Drug Discov. 2005 Feb;4(2):93-4.
|
| 4 |
Tinidazole. 2021 Mar 17. Drugs and Lactation Database (LactMed?) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006C.
|
| 5 |
Tinidazole (Tindamax)--a new option for treatment of bacterial vaginosis. Med Lett Drugs Ther. 2007 Sep 10;49(1269):73-4.
|
| 6 |
Tinidazole (Tindamax) - a new anti-protozoal drug. Med Lett Drugs Ther. 2004 Aug 30;46(1190):70-2.
|
| 7 |
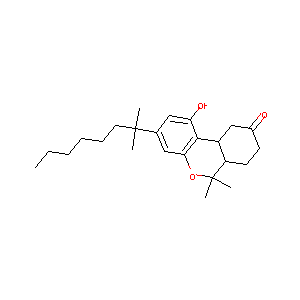
Novel 1',1'-chain substituted hexahydrocannabinols: 9-hydroxy-3-(1-hexyl-cyclobut-1-yl)-hexahydrocannabinol (AM2389) a highly potent cannabinoid r... J Med Chem. 2010 Oct 14;53(19):6996-7010.
|
| 8 |
Cloning and pharmacological characterization of the dog cannabinoid CB?receptor. Eur J Pharmacol. 2011 Nov 1;669(1-3):24-31. doi: 10.1016/j.ejphar.2011.08.002. Epub 2011 Aug 19.
|
| 9 |
FDA label of Tinidazole. The 2020 official website of the U.S. Food and Drug Administration.
|
| 10 |
Overexpression, isotopic labeling, and spectral characterization of Enterobacter cloacae nitroreductase. Protein Expr Purif. 1998 Jun;13(1):53-60.
|
| 11 |
Early identification of clinically relevant drug interactions with the human bile salt export pump (BSEP/ABCB11). Toxicol Sci. 2013 Dec;136(2):328-43.
|
|
|
|
|
|
|