| 1 |
ClinicalTrials.gov (NCT02416115) Anti Emetic Efficacy of Combination of Ramosetron and Premixture of Naloxone With Patient-controlled Analgesia
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1668).
|
| 3 |
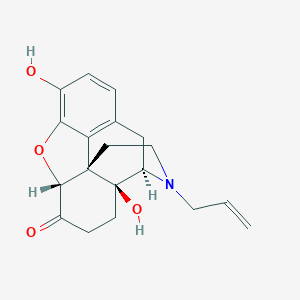
Naloxone FDA Label
|
| 4 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 5 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 6 |
Current and emerging pharmacological approaches for treating diarrhea-predominant irritable bowel syndrome. Expert Opin Pharmacother. 2020 Jan;21(1):63-71.
|
| 7 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2301).
|
| 8 |
ClinicalTrials.gov (NCT01225237) A Study to Evaluate Efficacy of Ramosetron on Diarrhea-predominant Irritable Bowel Syndrome (IBS) in Male Patients. U.S. National Institutes of Health.
|
| 9 |
Customised in vitro model to detect human metabolism-dependent idiosyncratic drug-induced liver injury. Arch Toxicol. 2018 Jan;92(1):383-399. doi: 10.1007/s00204-017-2036-4. Epub 2017 Jul 31.
|
| 10 |
OxyContin abuse and overdose. Postgrad Med. 2009 Mar;121(2):163-7.
|
| 11 |
beta-Endorphin and essential hypertension: importance of the clonidine-naloxone interaction. Acta Physiol Hung. 1985;65(2):217-26.
|
| 12 |
Opioid peptides attenuate blood pressure increase in acute respiratory failure. Peptides. 2001 Apr;22(4):631-7. doi: 10.1016/s0196-9781(01)00373-4.
|
| 13 |
Blood pressure and vasopressin in progressive autonomic failure. Response to postural stimulation, L-dopa and naloxone. Brain. 1983 Jun;106 (Pt 2):503-11. doi: 10.1093/brain/106.2.503.
|
| 14 |
Endogenous opioids modulate the cardiovascular response to mental stress. Psychoneuroendocrinology. 1990;15(3):185-92. doi: 10.1016/0306-4530(90)90029-9.
|
| 15 |
Differences in the opioid control of luteinizing hormone secretion between pathological and iatrogenic hyperprolactinemic states. J Clin Endocrinol Metab. 1987 Mar;64(3):508-12. doi: 10.1210/jcem-64-3-508.
|
| 16 |
Influence of naloxone on muscle sympathetic nerve activity, systemic and calf haemodynamics and ambulatory blood pressure after exercise in mild essential hypertension. J Hypertens. 1995 Apr;13(4):447-61.
|
| 17 |
Central opioid inhibition of neuroendocrine stress responses in pregnancy in the rat is induced by the neurosteroid allopregnanolone. J Neurosci. 2009 May 20;29(20):6449-60. doi: 10.1523/JNEUROSCI.0708-09.2009.
|
| 18 |
Chronic oxycodone induces integrated stress response in rat brain. BMC Neurosci. 2015 Sep 16;16:58. doi: 10.1186/s12868-015-0197-8.
|
| 19 |
Human carboxylesterase 1: from drug metabolism to drug discovery. Biochem Soc Trans. 2003 Jun;31(Pt 3):620-4. doi: 10.1042/bst0310620.
|
| 20 |
Interleukin-2-induced antinociception in morphine-insensitive rats. Acta Pharmacol Sin. 2002 Nov;23(11):981-4.
|
| 21 |
Inhibitory effect of YM060 on 5-HT3 receptor-mediated depolarization in colonic myenteric neurons of the guinea pig. Eur J Pharmacol. 1995 Sep 5;283(1-3):107-12.
|
| 22 |
Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77.
|
| 23 |
Contribution of P-glycoprotein to efflux of ramosetron, a 5-HT3 receptor antagonist, across the blood-brain barrier. J Pharm Pharmacol. 2002 Aug;54(8):1055-63.
|
| 24 |
Ondansetron, ramosetron, or palonosetron: which is a better choice of antiemetic to prevent postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy? Anesth Essays Res. 2011 Jul-Dec;5(2):182-6.
|
|
|
|
|
|
|