Details of the Drug
General Information of Drug (ID: DM3FXMA)
| Drug Name |
Naloxone
|
||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Nalone; Nalossone; Naloxona; Naloxonum; Narcan; Narcanti; Narcon; DBL Naloxone; Nalossone [DCIT]; Naloxone HCl; EN 1530base; DBL Naloxone (TN); L-Naloxone; N-Allylnoroxymorphone; Nalone (TN); Naloxona [INN-Spanish]; Naloxone (INN); Naloxone [INN:BAN]; Naloxonum [INN-Latin]; Narcan (TN); Narcanti (TN); N-Allyl-noroxymorphone; L-N-Allyl-14-hydroxynordihydromorphinone; L-N-Allyl-7,8-dihydro-14-hydroxynormorphinone; N-Allyl-4,5alpha-epoxy-3,14-dihydroxy-6-morphinanon; Morphinan-6-one, 17-allyl-4,5alpha-epoxy-3,14-dihydroxy-(8CI); Morphinan-6-one, 4,5-epoxy-3,14-dihydroxy-17-(2-propenyl)-, (5alpha)-(9CI); (-)-N-allyl-14-hydroxynordihydroxymorphinan-6-one; (-)-Naloxone; (5alpha)-3,14-dihydroxy-17-prop-2-en-1-yl-4,5-epoxymorphinan-6-one; (5alpha)-4,5-Epoxy-3,14-dihydroxy-17-(2-propenyl)morphinan-6-one; 1-N-Allyl-14-hydroxynordihydromorphinone; 1-N-Allyl-7,8-dihydro-14-hydroxynormorphinone; 12-Allyl-7,7a,8,9-tetrahydro-3,7a-dihydroxy-4aH-8,9c-iminoethanophenanthro(4,5-bcd)furanone; 12-Allyl-7,7a,8,9-tetrahydro-3,7a-dihydroxy-4aH-8,9c-iminoethanophenanthro[4,5-bcd]furan-5(6H)-one; 17-Allyl-4,5-alpha-epoxy-3,14-dihydroxymorphinan-6-one; 17-Allyl-4,5alpha-epoxy-3,14-dihydroxymorphinan-6-one; 17-allyl-3,14-dihydroxy-4,5alpha-epoxymorphinan-6-one; 3,14-dihydroxy-17-(prop-2-en-1-yl)-4,5alpha-epoxymorphinan-6-one
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antinarcotic Agents
|
||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
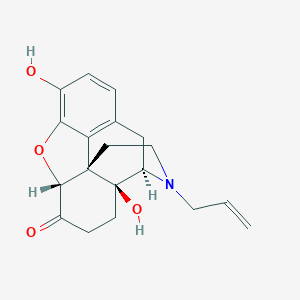 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 327.4 | |||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.1 | ||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Narcotic depression | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 6A7Z | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
