| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (NDA) 019578
|
| 3 |
ClinicalTrials.gov (NCT04347031) An Open Randomized Study of the Effectiveness of Mefloquine for the Treatment of Patients With COVID19. U.S. National Institutes of Health.
|
| 4 |
Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother. 2014 Aug;58(8):4885-93.
|
| 5 |
Mefloquine FDA Label
|
| 6 |
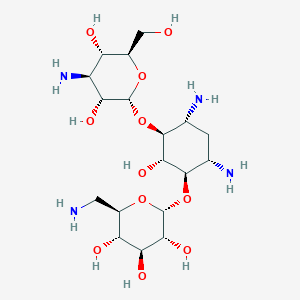
Kanamycin FDA Label
|
| 7 |
Novel agents in the management of Mycobacterium tuberculosis disease. Curr Med Chem. 2007;14(18):2000-8.
|
| 8 |
An ultrastructural study of the effects of mefloquine on malaria parasites. Am J Trop Med Hyg. 1987 Jan;36(1):9-14.
|
| 9 |
Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevance. Clin Pharmacol Ther. 2004 Jan;75(1):13-33.
|
| 10 |
Effect of rifampin on plasma concentrations of mefloquine in healthy volunteers. J Pharm Pharmacol. 2000 Oct;52(10):1265-9.
|
| 11 |
SsrA-mediated protein tagging in the presence of miscoding drugs and its physiological role in Escherichia coli. Genes Cells. 2002 Jul;7(7):629-38.
|
| 12 |
Relationship between antimicrobial resistance and aminoglycoside-modifying enzyme gene expressions in Acinetobacter baumannii. Chin Med J (Engl). 2005 Jan 20;118(2):141-5.
|
| 13 |
Cloning and expression of novel aminoglycoside phosphotransferase genes from Campylobacter and their role in the resistance to six aminoglycosides. Antimicrob Agents Chemother. 2017 Dec 21;62(1). pii: e01682-17.
|
| 14 |
Expression of Clostridium thermocellum endoglucanase gene in Lactobacillus gasseri and Lactobacillus johnsonii and characterization of the genetically modified probiotic lactobacilli. Curr Microbiol. 2000 Apr;40(4):257-63.
|
| 15 |
A plasmacytoid dendritic cell (CD123+/CD11c-) based assay system to predict contact allergenicity of chemicals. Toxicology. 2009 Oct 1;264(1-2):1-9. doi: 10.1016/j.tox.2009.07.021. Epub 2009 Aug 7.
|
|
|
|
|
|
|