| 1 |
ClinicalTrials.gov (NCT03154281) Evaluation of the Safety and Tolerability of Niraparib With Everolimus in Advanced Gynecologic Malignancies and Breast
|
| 2 |
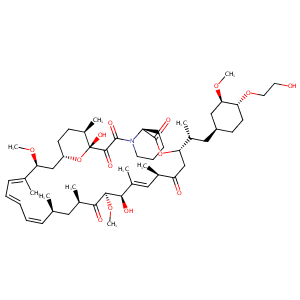
Everolimus FDA Label
|
| 3 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5889).
|
| 5 |
Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016 May;15(5):327-47.
|
| 6 |
ClinicalTrials.gov (NCT03602859) A Phase 3 Comparison of Platinum-based Therapy With TSR-042 and Niraparib Versus Standard of Care (SOC) Platinum-based Therapy as First-line Treatment of Stage III or IV Nonmucinous Epithelial Ovarian Cancer (FIRST). U.S. National Institutes of Health.
|
| 7 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 8 |
Mammalian target of rapamycin, its mode of action and clinical response in metastatic clear cell carcinoma. Gan To Kagaku Ryoho. 2009 Jul;36(7):1076-9.
|
| 9 |
Closer to the Site of Action: Everolimus Concentrations in Peripheral Blood Mononuclear Cells Correlate Well With Whole Blood Concentrations. Ther Drug Monit. 2015 Oct;37(5):675-80.
|
| 10 |
The evolving experience using everolimus in clinical transplantation. Transplant Proc. 2004 Mar;36(2 Suppl):495S-499S.
|
| 11 |
Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41.
|
| 12 |
Discovery of 2-{4-[(3S)-piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide (MK-4827): a novel oral poly(ADP-ribose)polymerase (PARP) inhibitor efficacious in BRCA-1 and -2 mutant tumors. J Med Chem. 2009 Nov 26;52(22):7170-85.
|
| 13 |
Summary of FDA-approved anticancer cytotoxic drugs at May 2019.
|
| 14 |
Autophagy up-regulated by MEK/ERK promotes the repair of DNA damage caused by aflatoxin B1. Toxicol Mech Methods. 2022 Feb;32(2):87-96. doi: 10.1080/15376516.2021.1968985. Epub 2021 Aug 26.
|
|
|
|
|
|
|