| 1 |
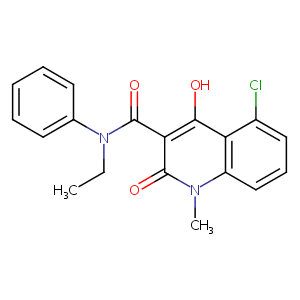
ClinicalTrials.gov (NCT01085097) A Study of Laquinimod in Participants With Systemic Lupus Erythematosus (SLE) Active Lupus Nephritis
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7639).
|
| 3 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 4 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 5 |
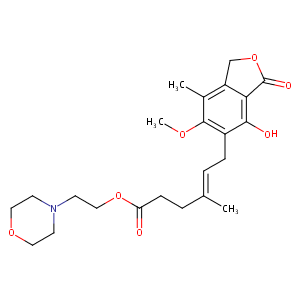
Mycophenolate mofetil FDA Label
|
| 6 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6831).
|
| 7 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 341).
|
| 8 |
High-Throughput Screening and Identification of Potent Broad-Spectrum Inhibitors of Coronaviruses. J Virol. 2019 May 29;93(12). pii: e00023-19.
|
| 9 |
Reduced astrocytic NF- B activation by laquinimod protects from cuprizone-induced demyelination. Acta Neuropathol. 2012 Sep;124(3):411-24.
|
| 10 |
Oral laquinimod treatment in multiple sclerosis. Neurologia. 2011 Mar;26(2):111-7.
|
| 11 |
Assessing anti-estrogenic effects of AHR ligands in primary human and rat endometrial epithelial cells. Reprod Toxicol. 2020 Sep;96:202-208. doi: 10.1016/j.reprotox.2020.07.003. Epub 2020 Jul 12.
|
| 12 |
Clinical pipeline report, company report or official report of Roche (2009).
|
| 13 |
Influence of SLCO1B1, 1B3, 2B1 and ABCC2 genetic polymorphisms on mycophenolic acid pharmacokinetics in Japanese renal transplant recipients. Eur J Clin Pharmacol. 2007 Dec;63(12):1161-9.
|
| 14 |
Influence of drug transporters and UGT polymorphisms on pharmacokinetics of phenolic glucuronide metabolite of mycophenolic acid in Japanese renal transplant recipients. Ther Drug Monit. 2008 Oct;30(5):559-64.
|
| 15 |
PharmGKB summary: mycophenolic acid pathway. Pharmacogenet Genomics. 2014 Jan;24(1):73-9.
|
| 16 |
Characterization of rat intestinal microsomal UDP-glucuronosyltransferase activity toward mycophenolic acid. Drug Metab Dispos. 2006 Sep;34(9):1632-9.
|
| 17 |
The evolution of population pharmacokinetic models to describe the enterohepatic recycling of mycophenolic acid in solid organ transplantation and autoimmune disease. Clin Pharmacokinet. 2011 Jan;50(1):1-24.
|
| 18 |
[Pharmacology of mycophenolate mofetil: recent data and clinical consequences]. Nephrologie. 2001;22(7):331-7.
|
|
|
|
|
|
|