Details of the Drug
General Information of Drug (ID: DM3IWS8)
| Drug Name |
Laquinimod
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Laquinimod; 248281-84-7; 5-CHLORO-N-ETHYL-4-HYDROXY-1-METHYL-2-OXO-N-PHENYL-1,2-DIHYDROQUINOLINE-3-CARBOXAMIDE; ABR-215062; ABR 215062; 5-Chloro-4-hydroxy-1-methyl-2-oxo-1,2-dihydro-quinoline-3-carboxylic acid ethyl-phenyl-amide; UNII-908SY76S4G; CIVENTICHEM CV-4057; Laquinimod (ABR-215062); 908SY76S4G; N-Ethyl-N-phenyl-1,2-dihydro-4-hydroxy-5-chloro-1-methyl-2-oxoquinoline-3-carboxamide; 5-chloro-n-ethyl-2-hydroxy-1-methyl-4-oxo-n-phenyl-1,4-dihydroquinoline-3-carboxamide; C19H17ClN2O3
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
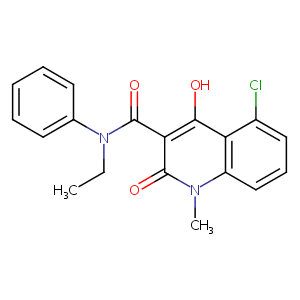 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 356.8 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.7 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||||||||||
| ADMET Property | |||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Lupus | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 4A40 | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
