| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7472).
|
| 3 |
Antibodies and venom peptides: new modalities for ion channels. Nat Rev Drug Discov. 2019 May;18(5):339-357.
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5210).
|
| 5 |
2008 FDA drug approvals. Nat Rev Drug Discov. 2009 Feb;8(2):93-6.
|
| 6 |
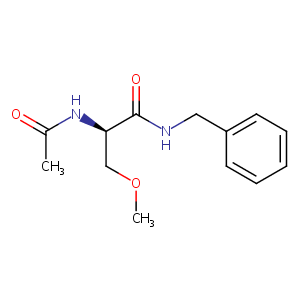
Lacosamide: what can be expected from the next new antiepileptic drug? Epilepsy Curr. 2009 Sep-Oct;9(5):133-4.
|
| 7 |
Lacosamide therapeutic monitoring in patients with epilepsy: effect of concomitant antiepileptic drugs. Ther Drug Monit. 2013 Dec;35(6):849-52.
|
| 8 |
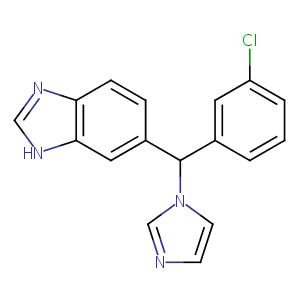
Pharmacophore modeling strategies for the development of novel nonsteroidal inhibitors of human aromatase (CYP19). Bioorg Med Chem Lett. 2010 May 15;20(10):3050-64.
|
| 9 |
Novel azolyl-(phenylmethyl)]aryl/heteroarylamines: potent CYP26 inhibitors and enhancers of all-trans retinoic acid activity in neuroblastoma cells. Bioorg Med Chem. 2008 Sep 1;16(17):8301-13.
|
| 10 |
Discovery of inhibitors of MCF-7 tumor cell adhesion to endothelial cells and investigation on their mode of action. Arch Pharm (Weinheim). 2004 Dec;337(12):687-94. doi: 10.1002/ardp.200400622.
|
|
|
|
|
|
|