| 1 |
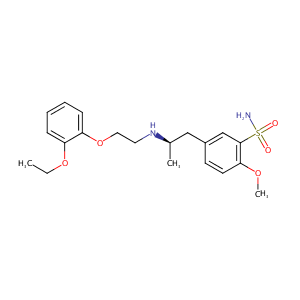
ClinicalTrials.gov (NCT02715024) Study to Evaluate the Clinical Efficacy and Safety of Tamsulosin Alone or in Combination With Solifenacin for the Treatment in Men With Lower Urinary Tract Symptoms Including Overactive Bladder Symptoms
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 488).
|
| 3 |
Tamsulosin FDA Label
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7483).
|
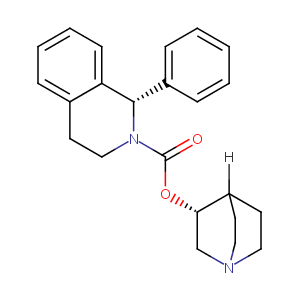
| 5 |
Solifenacin FDA Label
|
| 6 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services.
|
| 7 |
Identification of cytochrome P450 isozymes involved in metabolism of the alpha1-adrenoceptor blocker tamsulosin in human liver microsomes. Xenobiotica. 1998 Oct;28(10):909-22.
|
| 8 |
Cell membrane chromatography competitive binding analysis for characterization of 1A adrenoreceptor binding interactions. Anal Bioanal Chem. 2011 Jul;400(10):3625-33. doi: 10.1007/s00216-011-5026-z. Epub 2011 May 5.
|
| 9 |
Evaluating the Role of Multidrug Resistance Protein 3 (MDR3) Inhibition in Predicting Drug-Induced Liver Injury Using 125 Pharmaceuticals. Chem Res Toxicol. 2017 May 15;30(5):1219-1229. doi: 10.1021/acs.chemrestox.7b00048. Epub 2017 May 4.
|
| 10 |
Comparison of muscarinic receptor selectivity of solifenacin and oxybutynin in the bladder and submandibular gland of muscarinic receptor knockout ... Eur J Pharmacol. 2009 Aug 1;615(1-3):201-6.
|
| 11 |
Clinical pharmacokinetics and pharmacodynamics of solifenacin. Clin Pharmacokinet. 2009;48(5):281-302.
|
| 12 |
ClinicalTrials.gov (NCT02180997) Clinical Trial to Investigate the Pharmacokinetic Drug Interaction Between Solifenacin and Tamsulosin
|
|
|
|
|
|
|