| Molecular Interaction Atlas (MIA) |
|
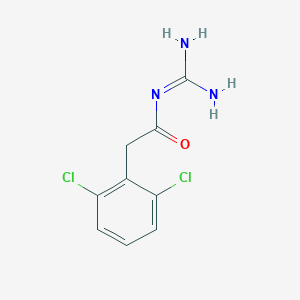
| Indication(s) of Guanfacine |
| Disease Entry |
ICD 11 |
Status |
REF |
| Attention deficit hyperactivity disorder |
6A05.Z
|
Approved |
[2] |
| Alzheimer disease |
8A20
|
Phase 3 |
[3] |
|
|
|
Guanfacine Interacts with 1 DTT Molecule(s)
| DTT Name |
DTT ID |
UniProt ID |
Mode of Action |
REF |
|
Adrenergic receptor alpha-2A (ADRA2A)
|
TTWG9A4
|
ADA2A_HUMAN
|
Agonist
|
[5] |
| ------------------------------------------------------------------------------------ |
|
|
|
|
|
|
Guanfacine Interacts with 1 DTP Molecule(s)
| DTP Name |
DTP ID |
UniProt ID |
Mode of Action |
REF |
|
P-glycoprotein 1 (ABCB1)
|
DTUGYRD
|
MDR1_HUMAN
|
Substrate
|
[6] |
| ------------------------------------------------------------------------------------ |
|
|
|
|
|
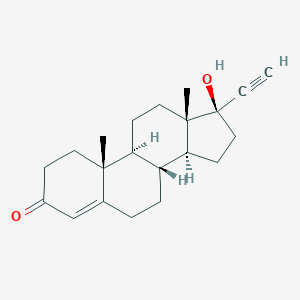
| Indication(s) of ETHISTERONE |
| Disease Entry |
ICD 11 |
Status |
REF |
| Discovery agent |
N.A.
|
Investigative |
[4] |
|
|
|
ETHISTERONE Interacts with 1 DTT Molecule(s)
| DTT Name |
DTT ID |
UniProt ID |
Mode of Action |
REF |
|
Tyrosinase (TYR)
|
TTULVH8
|
TYRO_HUMAN
|
Inhibitor
|
[4] |
| ------------------------------------------------------------------------------------ |
|
|
|
|
|
|
ETHISTERONE Interacts with 3 DOT Molecule(s)
| DOT Name |
DOT ID |
UniProt ID |
Mode of Action |
REF |
|
Serine/threonine-protein kinase Sgk1 (SGK1)
|
OT301T1U
|
SGK1_HUMAN
|
Increases Expression
|
[7] |
|
Myb-related protein A (MYBL1)
|
OTBJMC2P
|
MYBA_HUMAN
|
Increases Expression
|
[7] |
|
Protein GREB1 (GREB1)
|
OTU6ZA26
|
GREB1_HUMAN
|
Increases Expression
|
[7] |
| ------------------------------------------------------------------------------------ |
|
|
|
|
|
|
|
|
|
|
|
|