Details of the Drug
General Information of Drug (ID: DMPN5IH)
| Drug Name |
Guanfacine
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
GUANFACINE; Tenex; Guanfacinum [INN-Latin]; Guanfacina [INN-Spanish]; Estulic; Intuniv; N-amidino-2-(2,6-dichlorophenyl)acetamide; CHEBI:5558; INJOMKTZOLKMBF-UHFFFAOYSA-N; SPD 503; C9H9Cl2N3O; N-carbamimidoyl-2-(2,6-dichlorophenyl)acetamide; N-(diaminomethylidene)-2-(2,6-dichlorophenyl)acetamide; Guanfacine Monohydrochloride; NCGC00015469-05; Lon798; Guanfacine (INN); Estulic (TN); Tenex (Salt/Mix); Estulic (Salt/Mix); Tocris-1030; Prestwick1_000339; Prestwick3_000339; Prestwick2_000339; Prestwick0_000339; Lopac-G-1043
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Structure |
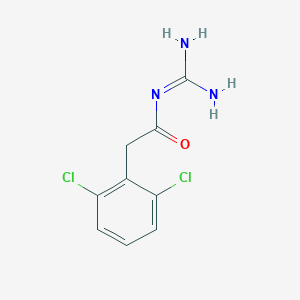 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 246.09 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||||||
| Rotatable Bond Count | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 1 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
