| 1 |
ClinicalTrials.gov (NCT03173560) Trial to Assess Safety and Efficacy of Lenvatinib (18 mg vs. 14 mg) in Combination With Everolimus in Participants With Renal Cell Carcinoma
|
| 2 |
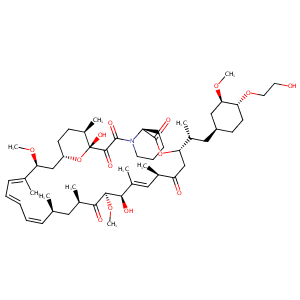
Everolimus FDA Label
|
| 3 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5889).
|
| 5 |
Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016 May;15(5):327-47.
|
| 6 |
FDA Approved Drug Products from FDA Official Website. 2018. Application Number: (ANDA) 208627.
|
| 7 |
Lenvatinib FDA Label
|
| 8 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 9 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 10 |
Emerging drugs for ovarian cancer. Expert Opin Emerg Drugs. 2008 Sep;13(3):523-36.
|
| 11 |
Mammalian target of rapamycin, its mode of action and clinical response in metastatic clear cell carcinoma. Gan To Kagaku Ryoho. 2009 Jul;36(7):1076-9.
|
| 12 |
Closer to the Site of Action: Everolimus Concentrations in Peripheral Blood Mononuclear Cells Correlate Well With Whole Blood Concentrations. Ther Drug Monit. 2015 Oct;37(5):675-80.
|
| 13 |
The evolving experience using everolimus in clinical transplantation. Transplant Proc. 2004 Mar;36(2 Suppl):495S-499S.
|
| 14 |
Influence of CYP3A4/5 and ABC transporter polymorphisms on lenvatinib plasma trough concentrations in Japanese patients with thyroid cancer. Sci Rep. 2019 Apr 1;9(1):5404.
|
| 15 |
ClinicalTrials.gov (NCT02811861) Lenvatinib/Everolimus or Lenvatinib/Pembrolizumab Versus Sunitinib Alone as Treatment of Advanced Renal Cell Carcinoma
|
|
|
|
|
|
|