| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
Blockade of KCa3.1: A novel target to treat TGF-1 induced conjunctival fibrosis.Exp Eye Res. 2018 Feb;167:140-144.
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7470).
|
| 4 |
Clinical pipeline report, company report or official report of Eisai.
|
| 5 |
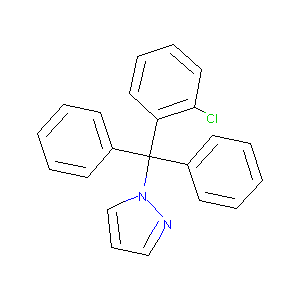
Inhibitors of potassium channels KV1.3 and IK-1 as immunosuppressants. Bioorg Med Chem Lett. 2009 Apr 15;19(8):2299-304.
|
| 6 |
Blockade of the intermediate-conductance calcium-activated potassium channel as a new therapeutic strategy for restenosis. Circulation. 2003 Sep 2;108(9):1119-25.
|
| 7 |
The intermediate conductance Ca2+-activated K+ channel inhibitor TRAM-34 stimulates proliferation of breast cancer cells via activation of oestrogen receptors. Br J Pharmacol. 2010 Feb 1;159(3):650-8. doi: 10.1111/j.1476-5381.2009.00557.x. Epub 2009 Dec 24.
|
| 8 |
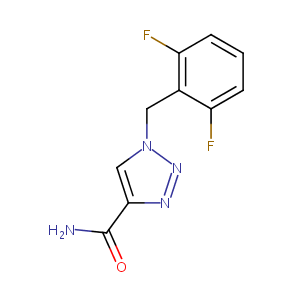
Emerging drugs for epilepsy. Expert Opin Emerg Drugs. 2007 Sep;12(3):407-22.
|
| 9 |
Investigation of the metabolism of rufinamide and its interaction with valproate. Drug Metab Lett. 2011 Dec;5(4):280-9.
|
|
|
|
|
|
|