| 1 |
ClinicalTrials.gov (NCT02732119) Study of Ribociclib With Everolimus + Exemestane in HR+ HER2- Locally Advanced/Metastatic Breast Cancer Post Progression on CDK 4/6 Inhibitor.
|
| 2 |
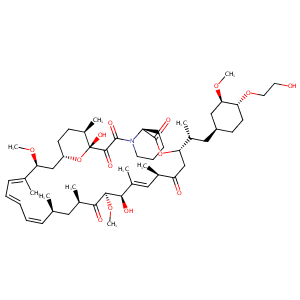
Everolimus FDA Label
|
| 3 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5889).
|
| 5 |
Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016 May;15(5):327-47.
|
| 6 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7073).
|
| 7 |
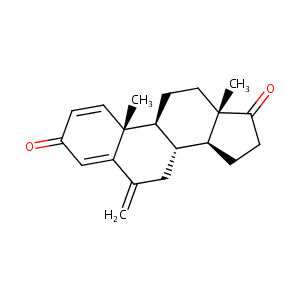
Exemestane FDA Label
|
| 8 |
Mammalian target of rapamycin, its mode of action and clinical response in metastatic clear cell carcinoma. Gan To Kagaku Ryoho. 2009 Jul;36(7):1076-9.
|
| 9 |
Closer to the Site of Action: Everolimus Concentrations in Peripheral Blood Mononuclear Cells Correlate Well With Whole Blood Concentrations. Ther Drug Monit. 2015 Oct;37(5):675-80.
|
| 10 |
The evolving experience using everolimus in clinical transplantation. Transplant Proc. 2004 Mar;36(2 Suppl):495S-499S.
|
| 11 |
Aromatase inhibitors--theoretical concept and present experiences in the treatment of endometriosis. Zentralbl Gynakol. 2003 Jul-Aug;125(7-8):247-51.
|
| 12 |
In vitro metabolism of exemestane by hepatic cytochrome P450s: impact of nonsynonymous polymorphisms on formation of the active metabolite 17beta-dihydroexemestane. Pharmacol Res Perspect. 2017 Apr 27;5(3):e00314.
|
| 13 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
| 14 |
Characterization of the weak estrogen receptor alpha agonistic activity of exemestane. Breast Cancer Res Treat. 2009 Aug;116(3):461-70. doi: 10.1007/s10549-008-0151-x. Epub 2008 Aug 3.
|
| 15 |
Effects of aromatase inhibitors on human osteoblast and osteoblast-like cells: a possible androgenic bone protective effects induced by exemestane. Bone. 2007 Apr;40(4):876-87. doi: 10.1016/j.bone.2006.11.029. Epub 2006 Dec 28.
|
| 16 |
Identifying environmental chemicals as agonists of the androgen receptor by using a quantitative high-throughput screening platform. Toxicology. 2017 Jun 15;385:48-58. doi: 10.1016/j.tox.2017.05.001. Epub 2017 May 4.
|
| 17 |
Aromatase inhibition: translation into a successful therapeutic approach. Clin Cancer Res. 2005 Apr 15;11(8):2809-21. doi: 10.1158/1078-0432.CCR-04-2187.
|
| 18 |
Aromatase inhibitors: cellular and molecular effects. J Steroid Biochem Mol Biol. 2005 May;95(1-5):83-9. doi: 10.1016/j.jsbmb.2005.04.010.
|
| 19 |
ClinicalTrials.gov (NCT00863655) Everolimus in Combination With Exemestane in the Treatment of Postmenopausal Women With Estrogen Receptor Positive Locally Advanced or Metastatic Breast Cancer Who Are Refractory to Letrozole or Anastrozole
|
| 20 |
ClinicalTrials.gov (NCT01626222) 4EVER - Efficacy, Safety, Health Economics, Translational Research of Postmenopausal Women With Estrogen Receptor Positive Locally Advanced or Metastatic Breast Cancer
|
| 21 |
ClinicalTrials.gov (NCT02077933) Study of Safety and Efficacy of Alpelisib With Everolimus or Alpelisib With Everolimus and Exemestane in Advanced Breast Cancer Patients, Renal Cell Cancer and Pancreatic Tumors
|
|
|
|
|
|
|