| 1 |
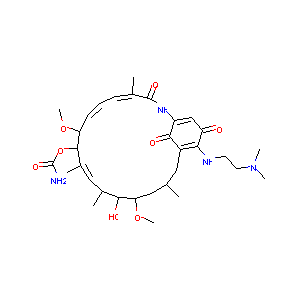
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
ClinicalTrials.gov (NCT00089271) 17-DMAG in Treating Patients With Metastatic or Unresectable Solid Tumors or Lymphomas. U.S. National Institutes of Health.
|
| 3 |
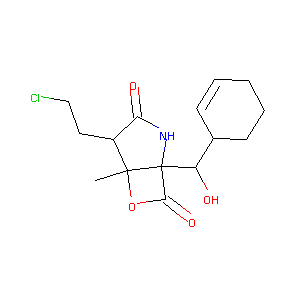
ClinicalTrials.gov (NCT03345095) A Phase III Trial of With Marizomib in Patients With Newly Diagnosed Glioblastoma (MIRAGE). U.S. National Institutes of Health.
|
| 4 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 5 |
Clinical pipeline report, company report or official report of Triphase Accelerator .
|
| 6 |
Stage 1 testing and pharmacodynamic evaluation of the HSP90 inhibitor alvespimycin (17-DMAG, KOS-1022) by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008 Jul;51(1):34-41.
|
| 7 |
Marizomib, a proteasome inhibitor for all seasons: preclinical profile and a framework for clinical trials. Curr Cancer Drug Targets. 2011 Mar;11(3):254-84.
|
|
|
|
|
|
|