| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
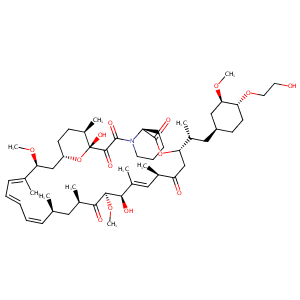
Everolimus FDA Label
|
| 3 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5889).
|
| 5 |
Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016 May;15(5):327-47.
|
| 6 |
ClinicalTrials.gov (NCT02115659) Triptolide-Containing Formulation as Treatment for Autosomal Dominant Polycystic Kidney Disease (ADPKD). U.S. National Institutes of Health.
|
| 7 |
Mammalian target of rapamycin, its mode of action and clinical response in metastatic clear cell carcinoma. Gan To Kagaku Ryoho. 2009 Jul;36(7):1076-9.
|
| 8 |
Closer to the Site of Action: Everolimus Concentrations in Peripheral Blood Mononuclear Cells Correlate Well With Whole Blood Concentrations. Ther Drug Monit. 2015 Oct;37(5):675-80.
|
| 9 |
The evolving experience using everolimus in clinical transplantation. Transplant Proc. 2004 Mar;36(2 Suppl):495S-499S.
|
| 10 |
Triptolide impairs thioredoxin system by suppressing Notch1-mediated PTEN/Akt/Txnip signaling in hepatocytes. Toxicol Lett. 2019 Jan;300:105-115. doi: 10.1016/j.toxlet.2018.10.024. Epub 2018 Oct 28.
|
| 11 |
Functional p53 is required for triptolide-induced apoptosis and AP-1 and nuclear factor-kappaB activation in gastric cancer cells. Oncogene. 2001 Nov 29;20(55):8009-18.
|
| 12 |
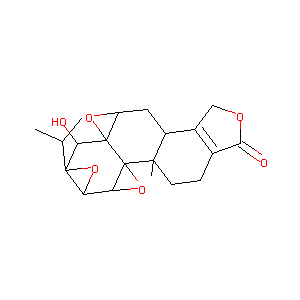
Preclinical pharmacokinetics of triptolide: a potential antitumor drug. Curr Drug Metab. 2019;20(2):147-154.
|
| 13 |
Variable p53/Nrf2 crosstalk contributes to triptolide-induced hepatotoxic process. Toxicol Lett. 2023 Apr 15;379:67-75. doi: 10.1016/j.toxlet.2023.03.011. Epub 2023 Mar 28.
|
| 14 |
Triptolide alters histone H3K9 and H3K27 methylation state and induces G0/G1 arrest and caspase-dependent apoptosis in multiple myeloma in vitro. Toxicology. 2010 Jan 12;267(1-3):70-9. doi: 10.1016/j.tox.2009.10.023. Epub 2009 Oct 29.
|
| 15 |
Triptolide inhibits the growth and metastasis of solid tumors. Mol Cancer Ther. 2003 Jan;2(1):65-72.
|
| 16 |
Interleukin-6-independent expression of glucocorticoid receptor is upregulated by triptolide in multiple myeloma. Leuk Lymphoma. 2009 May;50(5):802-8. doi: 10.1080/10428190902801838.
|
| 17 |
High-content analysis boosts identification of the initial cause of triptolide-induced hepatotoxicity. J Appl Toxicol. 2019 Sep;39(9):1337-1347. doi: 10.1002/jat.3821. Epub 2019 Jun 19.
|
| 18 |
Upregulation of ICAM-1 expression in bronchial epithelial cells by airway secretions in bronchiectasis. Respir Med. 2008 Feb;102(2):287-98. doi: 10.1016/j.rmed.2007.08.013. Epub 2007 Oct 10.
|
| 19 |
Catalpol and panax notoginseng saponins synergistically alleviate triptolide-induced hepatotoxicity through Nrf2/ARE pathway. Toxicol In Vitro. 2019 Apr;56:141-149. doi: 10.1016/j.tiv.2019.01.016. Epub 2019 Jan 23.
|
| 20 |
Differential expression and oxidation of MKP-1 modulates TNF-alpha gene expression. Am J Respir Cell Mol Biol. 2007 Sep;37(3):366-74. doi: 10.1165/rcmb.2006-0268OC. Epub 2007 May 16.
|
| 21 |
[Study of triptolide-induced apoptosis in MUTZ-1 cells and its allied mechanism]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2005 Jun;13(3):434-9.
|
| 22 |
Establishment of hypoxia induction in an in vivo animal replacement model for experimental evaluation of pancreatic cancer. Oncol Rep. 2014 Jul;32(1):153-8. doi: 10.3892/or.2014.3196. Epub 2014 May 16.
|
| 23 |
Cytotoxicity of Triptolide and Triptolide loaded polymeric micelles in vitro. Toxicol In Vitro. 2011 Dec;25(8):1557-67. doi: 10.1016/j.tiv.2011.05.020. Epub 2011 May 27.
|
| 24 |
Heat shock protein 72 protects kidney proximal tubule cells from injury induced by triptolide by means of activation of the MEK/ERK pathway. Int J Toxicol. 2009 May-Jun;28(3):177-89. doi: 10.1177/1091581809337418.
|
|
|
|
|
|
|