| 1 |
ClinicalTrials.gov (NCT00077376) Trastuzumab, Ixabepilone, and Carboplatin in Treating Patients With HER2/Neu-Positive Metastatic Breast Cancer
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6824).
|
| 3 |
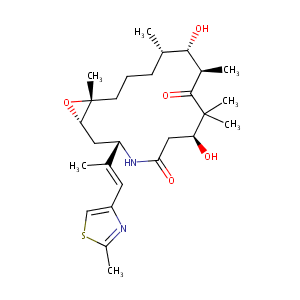
Ixabepilone FDA Label
|
| 4 |
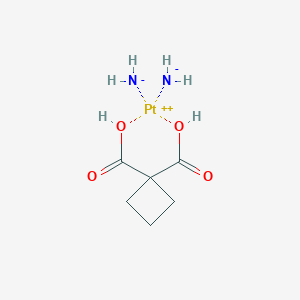
Carboplatin FDA Label
|
| 5 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7624).
|
| 6 |
Mullard A: 2010 FDA drug approvals. Nat Rev Drug Discov. 2011 Feb;10(2):82-5.
|
| 7 |
Ixabepilone, a novel microtubule-targeting agent for breast cancer, is a substrate for P-glycoprotein (P-gp/MDR1/ABCB1) but not breast cancer resistance protein (BCRP/ABCG2). J Pharmacol Exp Ther. 2011 May;337(2):423-32.
|
| 8 |
The effect of ketoconazole on the pharmacokinetics and pharmacodynamics of ixabepilone: a first in class epothilone B analogue in late-phase clinical development. Clin Cancer Res. 2008 May 1;14(9):2701-9.
|
| 9 |
Inhibition of carboplatin-induced DNA interstrand cross-link repair by gemcitabine in patients receiving these drugs for platinum-resistant ovarian cancer.Clin Cancer Res.2010 Oct 1;16(19):4899-905.
|
| 10 |
Overcoming platinum drug resistance with copper-lowering agents. Anticancer Res. 2013 Oct;33(10):4157-61.
|
| 11 |
PharmGKB: A worldwide resource for pharmacogenomic information. Wiley Interdiscip Rev Syst Biol Med. 2018 Jul;10(4):e1417. (ID: PA150642262)
|
| 12 |
ClinicalTrials.gov (NCT00028561) BMS-247550 Plus Carboplatin in Treating Patients With Recurrent or Refractory Solid Tumors
|
|
|
|
|
|
|