| 1 |
ClinicalTrials.gov (NCT03009981) A Study of Androgen Annihilation in High-Risk Biochemically Relapsed Prostate Cancer
|
| 2 |
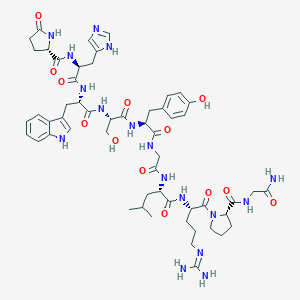
Gonadorelin FDA Label
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4391).
|
| 4 |
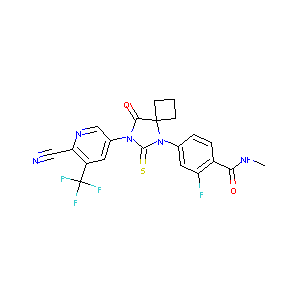
2018 FDA drug approvals.Nat Rev Drug Discov. 2019 Feb;18(2):85-89.
|
| 5 |
ClinicalTrials.gov (NCT01946204) A Study of ARN-509 in Men With Non-Metastatic Castration-Resistant Prostate Cancer. U.S. National Institutes of Health.
|
| 6 |
Use of cognitive behavior therapy for functional hypothalamic amenorrhea. Ann N Y Acad Sci. 2006 Dec;1092:114-29.
|
| 7 |
Ion-exchange chromatography: basic principles and application to the partial purification of soluble mammalian prolyl oligopeptidase. Methods Mol Biol. 2011;681:215-28.
|
| 8 |
Sleep deprivation changes thimet oligopeptidase (THOP1) expression and activity in rat brain. Heliyon. 2019 Nov 26;5(11):e02896.
|
| 9 |
Cell-specific activity of neprilysin 2 isoforms and enzymic specificity compared with neprilysin. Biochem J. 2002 May 1;363(Pt 3):697-705.
|
| 10 |
Apalutamide: first global approval. Drugs. 2018 Apr;78(6):699-705.
|
|
|
|
|
|
|