Details of the Drug
General Information of Drug (ID: DMAQ469)
| Drug Name |
Gonadorelin
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Dirigestran; Factrel; Fertagyl; Gonadorelina; Gonadorelinum; Hypocrine; Kryptocur; LFRH; LHFSHRH; Luforan; Luliberin; Lutal; Lutamin; Lutrefact; Relefact; Relisorm; Dirigestran Spofa; Gonadoliberin I; Gonadotropin releasing hormone; LHFSH Releasing Hormone; LUTEINIZING HORMONE; Lutrepulse KIT; MammalianGnRH; Relisorm l; Synthetic LRF; Synthetic gonadoliberin; HOE 471; AY-24031; FSH-Releasing Hormone; Fertagyl (TN); GnRH-I; Gonadorelin (INN); Gonadorelin [INN:BAN]; Gonadorelina [INN-Spanish]; Gonadorelinum [INN-Latin]; Gonadotropin-releasing factor; Gonadotropin-releasing hormone; Gonadotropin-releasing hormone I; Human LH-RH; LH-FSH Releasing Hormone; LH-Releasing hormone; Luteinizing hormone-releasing hormone; Mammalian LH-RH; Mammalian gonadotropin-releasing hormone; Ovine LH-RH; Ovine gonadotropin-releasing hormone; Porchine LH-RH; Porcine LH-releasing factor; Synthetic Gn-RH; Synthetic LH-FSH releasing hormone; Synthetic LH-RF; Synthetic LH-RH; Synthetic LH-releasing factor; Synthetic LH-releasing hormone; Synthetic gonadotropic hormone-releasing hormone; Synthetic gonadotropin-releasing hormone; LH-RH (swine); LH-Releasing factor (pig); LH-Releasing hormone (porcine); Luteinizing hormone-releasing factor (human); Luteinizing hormone-releasing factor (pig); Luteinizing hormone-releasing factor (rat); Luteinizing hormone-releasing factor (sheep); Luteinizing hormone-releasing factor (swine); Luteinizing hormone-releasing hormone (swin); Synthetic LH-FSH-RH; Synthetic decapeptide FSH/LH-RH; Follicle-stimulating hormone-releasing factor (pig); Gonadotropin, luteinizing hormone-releasing hormone, synthetic; Synthetic LH-RH/FSH-RH; Synthetic LH-releasing hormone/FSH-releasing hormone; PyroGlu-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly-NH2
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Fertility Agents
|
||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
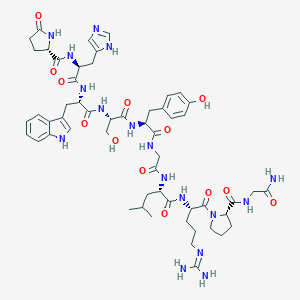 |
||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 5 | Molecular Weight (mw) | 1182.3 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -2.4 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 31 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 16 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 15 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
References
