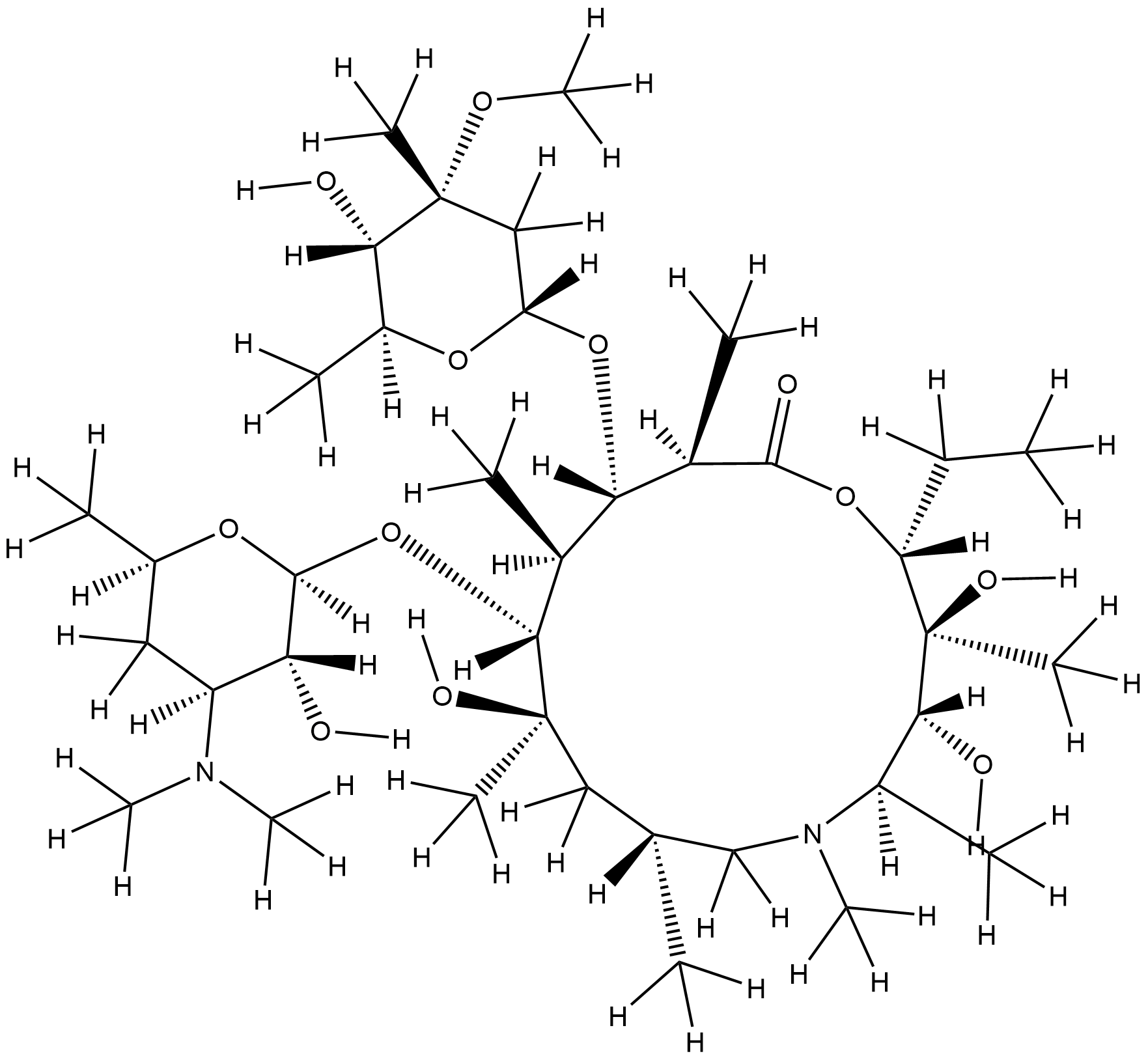
| 1 |
ClinicalTrials.gov (NCT00598962) Use of Azithromycin and Rifabutin Administered 3 Times Weekly for the Treatment of M. Avium Complex (MAC) Lung Disease
|
| 2 |
Novel agents in the management of Mycobacterium tuberculosis disease. Curr Med Chem. 2007;14(18):2000-8.
|
| 3 |
Azithromycin FDA Label
|
| 4 |
FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (ANDA) 065511.
|
| 5 |
ClinicalTrials.gov (NCT04332107) Azithromycin for COVID-19 Treatment in Outpatients Nationwide. U.S. National Institutes of Health.
|
| 6 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services.
|
| 7 |
The effect of multiple doses of ritonavir on the pharmacokinetics of rifabutin. Clin Pharmacol Ther. 1998 Apr;63(4):414-21.
|
|
|
|
|
|
|