| 1 |
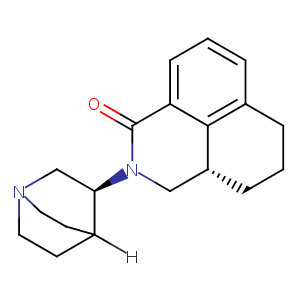
A phase III study evaluating the safety and efficacy of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting over repeated cycles of chemotherapy. Ann Oncol. 2014 Jul;25(7):1333-9.
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7486).
|
| 3 |
Palonosetron FDA Label
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5742).
|
| 5 |
Management of postoperative nausea and vomiting: focus on palonosetron. Ther Clin Risk Manag. 2009 Feb;5(1):21-34.
|
| 6 |
Pharmacokinetics, metabolism and excretion of intravenous [l4C]-palonosetron in healthy human volunteers. Biopharm Drug Dispos. 2004 Nov;25(8):329-37.
|
| 7 |
Cytochrome P450 2D6 metabolism and 5-hydroxytryptamine type 3 receptor antagonists for postoperative nausea and vomiting. Med Sci Monit. 2005 Oct;11(10):RA322-8.
|
| 8 |
Why are most phospholipidosis inducers also hERG blockers?. Arch Toxicol. 2017 Dec;91(12):3885-3895. doi: 10.1007/s00204-017-1995-9. Epub 2017 May 27.
|
| 9 |
Netupitant and palonosetron trigger NK1 receptor internalization in NG108-15 cells. Exp Brain Res. 2014 Aug;232(8):2637-44.
|
| 10 |
Complementary pharmacokinetic profiles of netupitant and palonosetron support the rationale for their oral fixed combination for the prevention of chemotherapy-induced nausea and vomiting. J Clin Pharmacol. 2019 Apr;59(4):472-487.
|
| 11 |
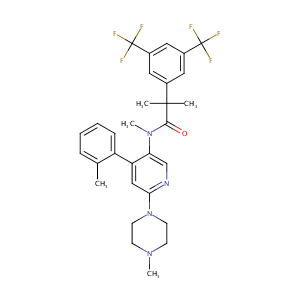
Effect of netupitant, a highly selective NK?receptor antagonist, on the pharmacokinetics of palonosetron and impact of the fixed dose combination of netupitant and palonosetron when coadministered with ketoconazole, rifampicin, and oral contraceptives. Support Care Cancer. 2013 Oct;21(10):2879-87.
|
| 12 |
ClinicalTrials.gov (NCT04669132) Efficacy of Olanzapine, Netupitant and Palonosetron in Controlling Nausea and Vomiting Associated With Highly Emetogenic Chemotherapy in Patients With Breast Cancer
|
| 13 |
ClinicalTrials.gov (NCT03204279) PK/PD Study of Netupitant and Palonosetron in Pediatric Patients for Prevention of Chemotherapy-induced Nausea and Vomiting
|
|
|
|
|
|
|