| 1 |
ClinicalTrials.gov (NCT00283686) HALT Progression of Polycystic Kidney Disease Study A
|
| 2 |
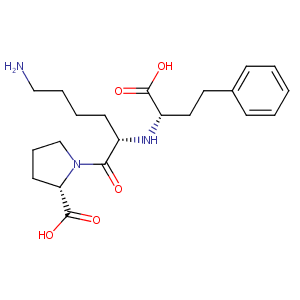
Lisinopril FDA Label
|
| 3 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 4 |
Coronavirus Disease 2019 (COVID-19) and Cardiovascular Disease: A Viewpoint on the Potential Influence of Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers on Onset and Severity of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J Am Heart Assoc. 2020 Apr 7;9(7):e016219.
|
| 5 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 592).
|
| 6 |
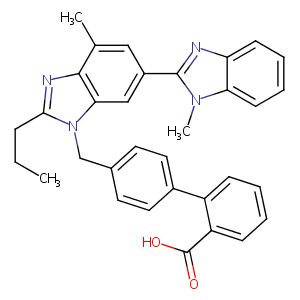
Telmisartan FDA Label
|
| 7 |
ClinicalTrials.gov (NCT04359953) Efficacy of Hydroxychloroquine, Telmisartan and Azithromycin on the Survival of Hospitalized Elderly Patients With COVID-19. U.S. National Institutes of Health.
|
| 8 |
Involvement of vascular angiotensin II-forming enzymes in the progression of aortic abdominal aneurysms in angiotensin II- infused ApoE-deficient m... J Atheroscler Thromb. 2009 Jun;16(3):164-71.
|
| 9 |
Peptide transporter substrate identification during permeability screening in drug discovery: comparison of transfected MDCK-hPepT1 cells to Caco-2 cells. Arch Pharm Res. 2007 Apr;30(4):507-18.
|
| 10 |
Targeted catalytic inactivation of angiotensin converting enzyme by lisinopril-coupled transition-metal chelates. J Am Chem Soc. 2012 Feb 22;134(7):3396-410. doi: 10.1021/ja208791f. Epub 2012 Feb 10.
|
| 11 |
Hemodynamic responses to converting enzyme inhibition in patients with renal disease. Am J Hypertens. 1989 Aug;2(8):599-603. doi: 10.1093/ajh/2.8.599.
|
| 12 |
Effects of lisinopril on cardiorespiratory, neuroendocrine, and renal function in patients with asymptomatic left ventricular dysfunction. Br Heart J. 1993 Jun;69(6):512-5. doi: 10.1136/hrt.69.6.512.
|
| 13 |
Different effects of angiotensin converting enzyme inhibitors on endothelin-1 and nitric oxide balance in human vascular endothelial cells: evidence of an oxidant-sensitive pathway. Mediators Inflamm. 2008;2008:305087. doi: 10.1155/2008/305087. Epub 2008 Dec 1.
|
| 14 |
Tissue availability of insulin-like growth factor I is inversely related to insulin resistance in essential hypertension: effects of angiotensin converting enzyme inhibition. J Hypertens. 1998 Jun;16(6):863-70. doi: 10.1097/00004872-199816060-00018.
|
| 15 |
Reduced bcl-2 concentrations in hypertensive patients after lisinopril or nifedipine administration. Am J Hypertens. 1999 Jan;12(1 Pt 1):73-5. doi: 10.1016/s0895-7061(98)00217-9.
|
| 16 |
Systems pharmacological analysis of drugs inducing stevens-johnson syndrome and toxic epidermal necrolysis. Chem Res Toxicol. 2015 May 18;28(5):927-34. doi: 10.1021/tx5005248. Epub 2015 Apr 3.
|
| 17 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
| 18 |
Deletion of angiotensin II type I receptor reduces hepatic steatosis. J Hepatol. 2009 Jun;50(6):1226-35.
|
| 19 |
Controversies of renin-angiotensin system inhibition during the COVID-19 pandemic. Nat Rev Nephrol. 2020 Apr 3.
|
| 20 |
Predominant contribution of OATP1B3 to the hepatic uptake of telmisartan, an angiotensin II receptor antagonist, in humans. Drug Metab Dispos. 2006 Jul;34(7):1109-15.
|
| 21 |
Establishment of a set of double transfectants coexpressing organic anion transporting polypeptide 1B3 and hepatic efflux transporters for the characterization of the hepatobiliary transport of telmisartan acylglucuronide. Drug Metab Dispos. 2008 Apr;36(4):796-805.
|
| 22 |
The impact of pharmacogenetics of metabolic enzymes and transporters on the pharmacokinetics of telmisartan in healthy volunteers. Pharmacogenet Genomics. 2011 Sep;21(9):523-30.
|
| 23 |
Characterization of in vitro glucuronidation clearance of a range of drugs in human kidney microsomes: comparison with liver and intestinal glucuronidation and impact of albumin. Drug Metab Dispos. 2012 Apr;40(4):825-35.
|
| 24 |
Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol Sci. 2010 Dec; 118(2):485-500.
|
| 25 |
Toxicological evaluation of acyl glucuronides utilizing half-lives, peptide adducts, and immunostimulation assays. Toxicol In Vitro. 2015 Dec 25;30(1 Pt B):241-9.
|
| 26 |
Inhibitory effects of antihypertensive drugs on human cytochrome P450 2J2 activity: Potent inhibition by azelnidipine and manidipine. Chem Biol Interact. 2019 Jun 1;306:1-9.
|
| 27 |
Metabolic effect of telmisartan and losartan in hypertensive patients with metabolic syndrome. Cardiovasc Diabetol. 2005 May 15;4:6. doi: 10.1186/1475-2840-4-6.
|
| 28 |
Improvement of endothelial function in patients with hypertension and type 2 diabetes after treatment with telmisartan. Hypertens Res. 2010 Aug;33(8):796-801. doi: 10.1038/hr.2010.107. Epub 2010 Jun 17.
|
| 29 |
Effect of telmisartan on ambulatory blood pressure monitoring, plasma brain natriuretic peptide, and oxidative status of serum albumin in hemodialysis patients. Hypertens Res. 2005 Dec;28(12):987-94. doi: 10.1291/hypres.28.987.
|
| 30 |
Telmisartan inhibits CD4-positive lymphocyte migration independent of the angiotensin type 1 receptor via peroxisome proliferator-activated receptor-gamma. Hypertension. 2008 Feb;51(2):259-66. doi: 10.1161/HYPERTENSIONAHA.107.099028. Epub 2007 Dec 24.
|
| 31 |
Telmisartan ameliorates lipopolysaccharide-induced innate immune response through peroxisome proliferator-activated receptor- activation in human monocytes. J Hypertens. 2012 Jan;30(1):87-96. doi: 10.1097/HJH.0b013e32834dde5f.
|
| 32 |
Comparison of the effects of telmisartan and olmesartan on home blood pressure, glucose, and lipid profiles in patients with hypertension, chronic heart failure, and metabolic syndrome. Hypertens Res. 2008 May;31(5):921-9. doi: 10.1291/hypres.31.921.
|
| 33 |
Telmisartan increases the permeability of endothelial cells through zonula occludens-1. Biol Pharm Bull. 2009 Mar;32(3):416-20. doi: 10.1248/bpb.32.416.
|
| 34 |
Differential regulation of tefluthrin and telmisartan on the gating charges of I(Na) activation and inactivation as well as on resurgent and persistent I(Na) in a pituitary cell line (GH(3)). Toxicol Lett. 2018 Mar 15;285:104-112. doi: 10.1016/j.toxlet.2018.01.002. Epub 2018 Jan 3.
|
| 35 |
ClinicalTrials.gov (NCT01885559) HALT Progression of Polycystic Kidney Disease Study B
|
|
|
|
|
|
|