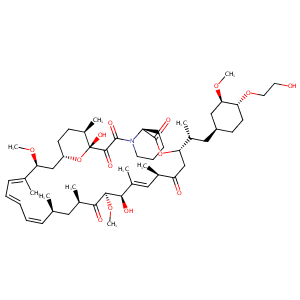
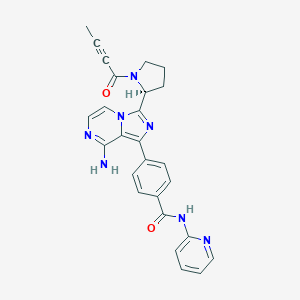
| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
Everolimus FDA Label
|
| 3 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5889).
|
| 5 |
Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016 May;15(5):327-47.
|
| 6 |
FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (NDA) 210259
|
| 7 |
2017 FDA drug approvals.Nat Rev Drug Discov. 2018 Feb;17(2):81-85.
|
| 8 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 9 |
ClinicalTrials.gov (NCT04346199) Acalabrutinib Study With Best Supportive Care Versus Best Supportive Care in Subjects Hospitalized With COVID-19.. U.S. National Institutes of Health.
|
| 10 |
Mammalian target of rapamycin, its mode of action and clinical response in metastatic clear cell carcinoma. Gan To Kagaku Ryoho. 2009 Jul;36(7):1076-9.
|
| 11 |
Closer to the Site of Action: Everolimus Concentrations in Peripheral Blood Mononuclear Cells Correlate Well With Whole Blood Concentrations. Ther Drug Monit. 2015 Oct;37(5):675-80.
|
| 12 |
The evolving experience using everolimus in clinical transplantation. Transplant Proc. 2004 Mar;36(2 Suppl):495S-499S.
|
| 13 |
AstraZeneca initiates CALAVI clinical trial with Calquence against COVID-19
|
| 14 |
FDA label of Acalabrutinib. The 2020 official website of the U.S. Food and Drug Administration.
|
| 15 |
Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci Immunol. 2020 Jun 5;5(48):eabd0110. doi: 10.1126/sciimmunol.abd0110. Epub 2020 Jun 5.
|
| 16 |
Functional assessment of the effects of CYP3A4 variants on acalabrutinib metabolism in vitro. Chem Biol Interact. 2021 Aug 25;345:109559. doi: 10.1016/j.cbi.2021.109559. Epub 2021 Jun 18.
|
|
|
|
|
|
|