| 1 |
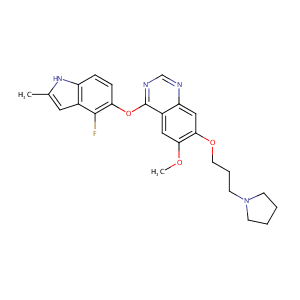
ClinicalTrials.gov (NCT00503204) Phase I : Cediranib in Combination With Lomustine Chemotherapy in Recurrent Malignant Brain Tumour
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7214).
|
| 3 |
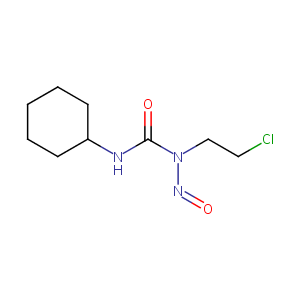
Lomustine FDA Label
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5664).
|
| 5 |
Synthesis and evaluation of ethylnitrosoureas of substituted naphthalimides as anticancer compounds. Acta Pol Pharm. 2007 Jan-Feb;64(1):27-33.
|
| 6 |
Characterization of DNA reactive and non-DNA reactive anticancer drugs by gene expression profiling. Mutat Res. 2007 Jun 1;619(1-2):16-29. doi: 10.1016/j.mrfmmm.2006.12.007. Epub 2007 Feb 8.
|
| 7 |
Enhanced in vitro invasiveness and drug resistance with altered gene expression patterns in a human lung carcinoma cell line after pulse selection with anticancer drugs. Int J Cancer. 2004 Sep 10;111(4):484-93. doi: 10.1002/ijc.20230.
|
| 8 |
High-content imaging-based BAC-GFP toxicity pathway reporters to assess chemical adversity liabilities. Arch Toxicol. 2017 Mar;91(3):1367-1383. doi: 10.1007/s00204-016-1781-0. Epub 2016 Jun 29.
|
| 9 |
Utilization of CDKN1A/p21 gene for class discrimination of DNA damage-induced clastogenicity. Toxicology. 2014 Jan 6;315:8-16. doi: 10.1016/j.tox.2013.10.009. Epub 2013 Nov 6.
|
| 10 |
Effect of common medications on the expression of SARS-CoV-2 entry receptors in liver tissue. Arch Toxicol. 2020 Dec;94(12):4037-4041. doi: 10.1007/s00204-020-02869-1. Epub 2020 Aug 17.
|
| 11 |
Translesion polymerase is upregulated by cancer therapeutics and confers anticancer drug resistance. Cancer Res. 2014 Oct 1;74(19):5585-96. doi: 10.1158/0008-5472.CAN-14-0953. Epub 2014 Aug 14.
|
| 12 |
Human osteosarcoma xenografts and their sensitivity to chemotherapy. Pathol Oncol Res. 2004;10(3):133-41. doi: 10.1007/BF03033741. Epub 2004 Sep 25.
|
| 13 |
The 1p-encoded protein stathmin and resistance of malignant gliomas to nitrosoureas. J Natl Cancer Inst. 2007 Apr 18;99(8):639-52. doi: 10.1093/jnci/djk135.
|
| 14 |
A comparison of physicochemical property profiles of marketed oral drugs and orally bioavailable anti-cancer protein kinase inhibitors in clinical development. Curr Top Med Chem. 2007;7(14):1408-22.
|
| 15 |
In vitro hepatic metabolism of cediranib, a potent vascular endothelial growth factor tyrosine kinase inhibitor: interspecies comparison and human enzymology. Drug Metab Dispos. 2010 Oct;38(10):1688-97.
|
|
|
|
|
|
|