Details of the Drug
General Information of Drug (ID: DMMWSUL)
| Drug Name |
Lomustine
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Belustine; CCNU; CINU; Cecenu; CeeNU; Chloroethylcyclohexylnitrosourea; Lomustina; Lomustinum; Bristol Myers Squibb Brand of Lomustine; CCNU [Chloroethyl nitrosoureas]; Cyclohexyl chloroethyl nitrosourea; Lomustine medac Brand; Medac Brand of Lomustine; Rhone Poulenc Rorer Brand of Lomustine; OR5087; RB 1509; SRI 2200; Bristol-Myers Squibb Brand of Lomustine; CeeNU (TN); Lomustina [INN-Spanish]; Lomustinum [INN-Latin]; NPFAPI-06; Rhone-Poulenc Rorer Brand of Lomustine; CeeNU, CCNU, Lomustine; Lomustine (USAN/INN); Lomustine [USAN:BAN:INN]; N-(2-Chloroethyl)-N'-cyclohexyl-N-nitrosourea; (Chloro-2-ethyl)-1-cyclohexyl-3-nitrosourea; (Cloro-2-etil)-1-cicloesil-3-nitrosourea; (Cloro-2-etil)-1-cicloesil-3-nitrosourea [Italian];1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea; 1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea [Chloroethyl nitrosoureas]; 1-(2-Chloroethyl)-3-cyclohexylnitrosourea
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
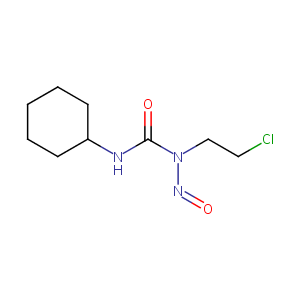 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 233.69 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.8 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Lomustine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7214). | ||||
|---|---|---|---|---|---|
| 2 | Lomustine FDA Label | ||||
| 3 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 4 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | Synthesis and evaluation of ethylnitrosoureas of substituted naphthalimides as anticancer compounds. Acta Pol Pharm. 2007 Jan-Feb;64(1):27-33. | ||||
| 7 | Effect of common medications on the expression of SARS-CoV-2 entry receptors in liver tissue. Arch Toxicol. 2020 Dec;94(12):4037-4041. doi: 10.1007/s00204-020-02869-1. Epub 2020 Aug 17. | ||||
| 8 | Enhanced in vitro invasiveness and drug resistance with altered gene expression patterns in a human lung carcinoma cell line after pulse selection with anticancer drugs. Int J Cancer. 2004 Sep 10;111(4):484-93. doi: 10.1002/ijc.20230. | ||||
| 9 | Utilization of CDKN1A/p21 gene for class discrimination of DNA damage-induced clastogenicity. Toxicology. 2014 Jan 6;315:8-16. doi: 10.1016/j.tox.2013.10.009. Epub 2013 Nov 6. | ||||
| 10 | High-content imaging-based BAC-GFP toxicity pathway reporters to assess chemical adversity liabilities. Arch Toxicol. 2017 Mar;91(3):1367-1383. doi: 10.1007/s00204-016-1781-0. Epub 2016 Jun 29. | ||||
| 11 | Translesion polymerase is upregulated by cancer therapeutics and confers anticancer drug resistance. Cancer Res. 2014 Oct 1;74(19):5585-96. doi: 10.1158/0008-5472.CAN-14-0953. Epub 2014 Aug 14. | ||||
| 12 | Human osteosarcoma xenografts and their sensitivity to chemotherapy. Pathol Oncol Res. 2004;10(3):133-41. doi: 10.1007/BF03033741. Epub 2004 Sep 25. | ||||
| 13 | The 1p-encoded protein stathmin and resistance of malignant gliomas to nitrosoureas. J Natl Cancer Inst. 2007 Apr 18;99(8):639-52. doi: 10.1093/jnci/djk135. | ||||
| 14 | Characterization of DNA reactive and non-DNA reactive anticancer drugs by gene expression profiling. Mutat Res. 2007 Jun 1;619(1-2):16-29. doi: 10.1016/j.mrfmmm.2006.12.007. Epub 2007 Feb 8. | ||||
| 15 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 16 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 17 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 18 | Johnson EJ, MacGowan AP, Potter MN, et al "Reduced absorption of oral ciprofloxacin after chemotherapy for haematological malignancy." J Antimicrob Chemother 25 (1990): 837-42. [PMID: 2373666] | ||||
| 19 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 20 | Product Information. Adcetris (brentuximab vedotin). Seattle Genetics Inc, Bothell, WA. | ||||
| 21 | Product Information. Kynamro (mipomersen). Genzyme Corporation, Cambridge, MA. | ||||
| 22 | Product Information. Arava (leflunomide). Hoechst Marion-Roussel Inc, Kansas City, MO. | ||||
| 23 | Product Information. Prolia (denosumab). Amgen USA, Thousand Oaks, CA. | ||||
| 24 | Al-Nawakil C, Willems L, Mauprivez C, et.al "Successful treatment of l-asparaginase-induced severe acute hepatotoxicity using mitochondrial cofactors." Leuk Lymphoma 55 (2014): 1670-4. [PMID: 24090500] | ||||
| 25 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 26 | Product Information. Vumerity (diroximel fumarate). Alkermes, Inc, Cambridge, MA. | ||||
| 27 | Product Information. Gilenya (fingolimod). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 28 | Product Information. Ocrevus (ocrelizumab). Genentech, South San Francisco, CA. | ||||
| 29 | Product Information. Synribo (omacetaxine). Teva Pharmaceuticals USA, North Wales, PA. | ||||
| 30 | Product Information. Arcalyst (rilonacept). Regeneron Pharmaceuticals Inc, Tarrytown, NY. | ||||
| 31 | Product Information. Cimzia (certolizumab). UCB Pharma Inc, Smyrna, GA. | ||||
| 32 | CDC. Centers for Disease Control and Prevention/ "Recommendations of the advisory committtee on immunization practices (ACIP): use of vaccines and immune globulins in persons with altered immunocompetence." MMWR Morb Mortal Wkly Rep 42(RR-04) (1993): 1-18. [PMID: 20300058] | ||||
