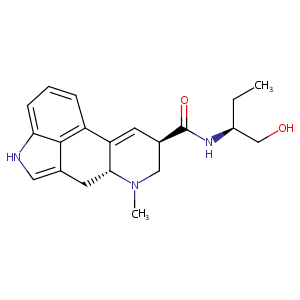
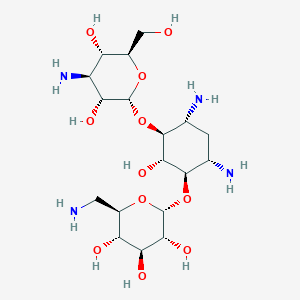
| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 3 |
Kanamycin FDA Label
|
| 4 |
Novel agents in the management of Mycobacterium tuberculosis disease. Curr Med Chem. 2007;14(18):2000-8.
|
| 5 |
Reinforcement in an in vitro analog of appetitive classical conditioning of feeding behavior in Aplysia: blockade by a dopamine antagonist. Learn Mem. 2005 May-Jun;12(3):216-20.
|
| 6 |
Modulation of cytochrome P450 metabolism by ergonovine and dihydroergotamine. Vet Hum Toxicol. 2003 Feb;45(1):6-9.
|
| 7 |
SsrA-mediated protein tagging in the presence of miscoding drugs and its physiological role in Escherichia coli. Genes Cells. 2002 Jul;7(7):629-38.
|
| 8 |
Relationship between antimicrobial resistance and aminoglycoside-modifying enzyme gene expressions in Acinetobacter baumannii. Chin Med J (Engl). 2005 Jan 20;118(2):141-5.
|
| 9 |
Cloning and expression of novel aminoglycoside phosphotransferase genes from Campylobacter and their role in the resistance to six aminoglycosides. Antimicrob Agents Chemother. 2017 Dec 21;62(1). pii: e01682-17.
|
| 10 |
Expression of Clostridium thermocellum endoglucanase gene in Lactobacillus gasseri and Lactobacillus johnsonii and characterization of the genetically modified probiotic lactobacilli. Curr Microbiol. 2000 Apr;40(4):257-63.
|
| 11 |
A plasmacytoid dendritic cell (CD123+/CD11c-) based assay system to predict contact allergenicity of chemicals. Toxicology. 2009 Oct 1;264(1-2):1-9. doi: 10.1016/j.tox.2009.07.021. Epub 2009 Aug 7.
|
|
|
|
|
|
|