| 1 |
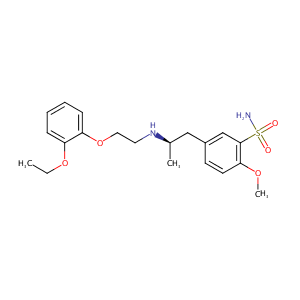
ClinicalTrials.gov (NCT02656173) A Phase 4 Study to Evaluate the Efficacy, Safety, and Tolerability of Mirabegron in Male Subjects With Overactive Bladder (OAB) Symptoms, While Taking the Alpha Blocker for Benign Prostatic Hypertrophy (BPH)
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 488).
|
| 3 |
Tamsulosin FDA Label
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7445).
|
| 5 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services.
|
| 6 |
Identification of cytochrome P450 isozymes involved in metabolism of the alpha1-adrenoceptor blocker tamsulosin in human liver microsomes. Xenobiotica. 1998 Oct;28(10):909-22.
|
| 7 |
Cell membrane chromatography competitive binding analysis for characterization of 1A adrenoreceptor binding interactions. Anal Bioanal Chem. 2011 Jul;400(10):3625-33. doi: 10.1007/s00216-011-5026-z. Epub 2011 May 5.
|
| 8 |
Evaluating the Role of Multidrug Resistance Protein 3 (MDR3) Inhibition in Predicting Drug-Induced Liver Injury Using 125 Pharmaceuticals. Chem Res Toxicol. 2017 May 15;30(5):1219-1229. doi: 10.1021/acs.chemrestox.7b00048. Epub 2017 May 4.
|
| 9 |
Nat Rev Drug Discov. 2013 Feb;12(2):87-90.
|
| 10 |
Role of cytochrome p450 isoenzymes 3A and 2D6 in the in vivo metabolism of mirabegron, a beta-3-adrenoceptor agonist. Clin Drug Investig. 2013 Jun;33(6):429-40.
|
| 11 |
ClinicalTrials.gov (NCT02095665) Ureteral Stent-related Pain and Mirabegron (SPAM) Trial
|
| 12 |
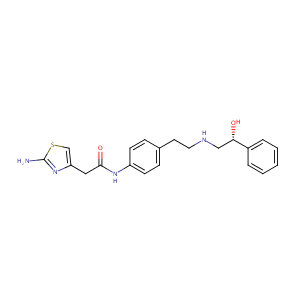
ClinicalTrials.gov (NCT01489696) A Study to Evaluate Cardiovascular Interactions Between Mirabegron and Tamsulosin
|
|
|
|
|
|
|