| 1 |
ClinicalTrials.gov (NCT02531932) Comparison of Single-Agent Carboplatin vs the Combination of Carboplatin and Everolimus for the Treatment of Advanced Triple-Negative Breast Cancer
|
| 2 |
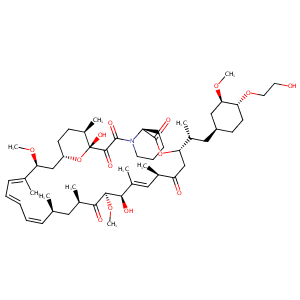
Everolimus FDA Label
|
| 3 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5889).
|
| 5 |
Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016 May;15(5):327-47.
|
| 6 |
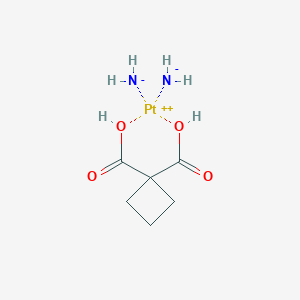
Carboplatin FDA Label
|
| 7 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7624).
|
| 8 |
Mammalian target of rapamycin, its mode of action and clinical response in metastatic clear cell carcinoma. Gan To Kagaku Ryoho. 2009 Jul;36(7):1076-9.
|
| 9 |
Closer to the Site of Action: Everolimus Concentrations in Peripheral Blood Mononuclear Cells Correlate Well With Whole Blood Concentrations. Ther Drug Monit. 2015 Oct;37(5):675-80.
|
| 10 |
The evolving experience using everolimus in clinical transplantation. Transplant Proc. 2004 Mar;36(2 Suppl):495S-499S.
|
| 11 |
Inhibition of carboplatin-induced DNA interstrand cross-link repair by gemcitabine in patients receiving these drugs for platinum-resistant ovarian cancer.Clin Cancer Res.2010 Oct 1;16(19):4899-905.
|
| 12 |
Overcoming platinum drug resistance with copper-lowering agents. Anticancer Res. 2013 Oct;33(10):4157-61.
|
| 13 |
PharmGKB: A worldwide resource for pharmacogenomic information. Wiley Interdiscip Rev Syst Biol Med. 2018 Jul;10(4):e1417. (ID: PA150642262)
|
| 14 |
ClinicalTrials.gov (NCT01014351) Study of Everolimus With Paclitaxel and Carboplatin in Patients With Metastatic Melanoma
|
| 15 |
ClinicalTrials.gov (NCT01127763) RAD001 Plus Carboplatin in Breast Cancer Patients
|
|
|
|
|
|
|