| 1 |
ClinicalTrials.gov (NCT01398267) A Pharmacodynamic/Pharmacokinetic Study of Aleglitazar in Patients With Type 2 Diabetes Mellitus on Treatment With Lisinopril
|
| 2 |
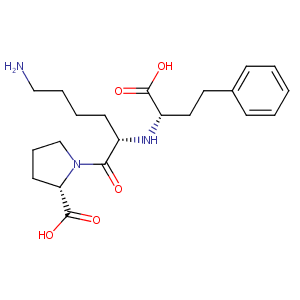
Lisinopril FDA Label
|
| 3 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 4 |
Coronavirus Disease 2019 (COVID-19) and Cardiovascular Disease: A Viewpoint on the Potential Influence of Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers on Onset and Severity of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J Am Heart Assoc. 2020 Apr 7;9(7):e016219.
|
| 5 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7405).
|
| 6 |
Involvement of vascular angiotensin II-forming enzymes in the progression of aortic abdominal aneurysms in angiotensin II- infused ApoE-deficient m... J Atheroscler Thromb. 2009 Jun;16(3):164-71.
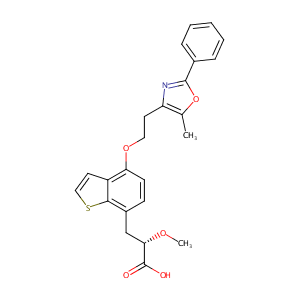
|
| 7 |
Peptide transporter substrate identification during permeability screening in drug discovery: comparison of transfected MDCK-hPepT1 cells to Caco-2 cells. Arch Pharm Res. 2007 Apr;30(4):507-18.
|
| 8 |
Targeted catalytic inactivation of angiotensin converting enzyme by lisinopril-coupled transition-metal chelates. J Am Chem Soc. 2012 Feb 22;134(7):3396-410. doi: 10.1021/ja208791f. Epub 2012 Feb 10.
|
| 9 |
Hemodynamic responses to converting enzyme inhibition in patients with renal disease. Am J Hypertens. 1989 Aug;2(8):599-603. doi: 10.1093/ajh/2.8.599.
|
| 10 |
Effects of lisinopril on cardiorespiratory, neuroendocrine, and renal function in patients with asymptomatic left ventricular dysfunction. Br Heart J. 1993 Jun;69(6):512-5. doi: 10.1136/hrt.69.6.512.
|
| 11 |
Different effects of angiotensin converting enzyme inhibitors on endothelin-1 and nitric oxide balance in human vascular endothelial cells: evidence of an oxidant-sensitive pathway. Mediators Inflamm. 2008;2008:305087. doi: 10.1155/2008/305087. Epub 2008 Dec 1.
|
| 12 |
Tissue availability of insulin-like growth factor I is inversely related to insulin resistance in essential hypertension: effects of angiotensin converting enzyme inhibition. J Hypertens. 1998 Jun;16(6):863-70. doi: 10.1097/00004872-199816060-00018.
|
| 13 |
Reduced bcl-2 concentrations in hypertensive patients after lisinopril or nifedipine administration. Am J Hypertens. 1999 Jan;12(1 Pt 1):73-5. doi: 10.1016/s0895-7061(98)00217-9.
|
| 14 |
Systems pharmacological analysis of drugs inducing stevens-johnson syndrome and toxic epidermal necrolysis. Chem Res Toxicol. 2015 May 18;28(5):927-34. doi: 10.1021/tx5005248. Epub 2015 Apr 3.
|
| 15 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
| 16 |
Clinical pipeline report, company report or official report of Roche (2009).
|
| 17 |
Exposure and response analysis of aleglitazar on cardiovascular risk markers and safety outcomes: an analysis of the AleCardio trial. Diabetes Obes Metab. 2020 Jan;22(1):30-38.
|
|
|
|
|
|
|