Details of the Drug Combinations
General Information of This Drug (ID: DM3IWS8)
| Drug Name | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Laquinimod; 248281-84-7; 5-CHLORO-N-ETHYL-4-HYDROXY-1-METHYL-2-OXO-N-PHENYL-1,2-DIHYDROQUINOLINE-3-CARBOXAMIDE; ABR-215062; ABR 215062; 5-Chloro-4-hydroxy-1-methyl-2-oxo-1,2-dihydro-quinoline-3-carboxylic acid ethyl-phenyl-amide; UNII-908SY76S4G; CIVENTICHEM CV-4057; Laquinimod (ABR-215062); 908SY76S4G; N-Ethyl-N-phenyl-1,2-dihydro-4-hydroxy-5-chloro-1-methyl-2-oxoquinoline-3-carboxamide; 5-chloro-n-ethyl-2-hydroxy-1-methyl-4-oxo-n-phenyl-1,4-dihydroquinoline-3-carboxamide; C19H17ClN2O3
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
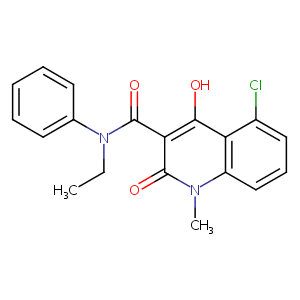 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
