Details of the Drug Combinations
General Information of This Drug (ID: DM3PD2C)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Eufor; Floxetine; Fluoxetin; Fluoxetina; Fluoxetinum; Fluval; Fontex; Portal; Pulvules; Reconcile; Reneuron; Fluoxetina [Spanish]; Fluoxetine Hcl; Prozac Weekly; Animex-On; Fluoxetina [INN-Spanish]; Fluoxetine (Prozac); Fluoxetine (TN); Fluoxetinum [INN-Latin]; Lilly-110140; Prozac (TN); Fluoxetine (USAN/INN); Fluoxetine [USAN:INN:BAN]; N-Methyl-gamma-(4-(trifluoromethyl)phenoxy)benzenepropanamine; Methyl({3-phenyl-3-[4-(trifluoromethyl)phenoxy]propyl})amine; N-Methyl-3-(p-trifluoromethylphenoxy)-3-phenylpropylamine; Dl-3-(p-Trifluoromethylphenoxy)-N-methyl-3-phenylpropylamine; Methyl-[3-phenyl-3-(4-trifluoromethyl-phenoxy)-propyl]-amine; N-methyl-3-phenyl-3-[4-(trifluoromethyl)phenoxy]propan-1-amine; N-methyl-3-phenyl-3-{[4-(trifluoromethyl)phenyl]oxy}propan-1-amine; (+-)-N-Methyl-3-phenyl-3-((alpha,alpha,alpha-trifluoro-p-tolyl)oxy)propylamine; (+-)-N-Methyl-gamma-(4-(trifluoromethyl)phenoxy)benzenepropanamine;(+/-)-Fluoxetine; (+/-)-N-Methyl-3-(p-trifluoromethylphenoxy)-3-phenylpropylamine; (+/-)-N-Methyl-3-p-(p-trifluoromethylphenoxy)-3-phenylpropylamine; (+/-)-N-Methyl-3-phenyl-3-[(alpha,alpha,alpha-trifluoro-p-tolyl)oxy]propylamine; (+/-)-N-Methyl-3-phenyl-3-[4-(trifluoromethyl)phenoxy]propylamine]; (+/-)-N-Methyl-gamma-[4-(trifluoromethyl)phenoxy]benzenepropanamine; 3-(p-Trifluoromethylphenoxy)-N-methyl-3-phenylpropylamine
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antidepressants
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
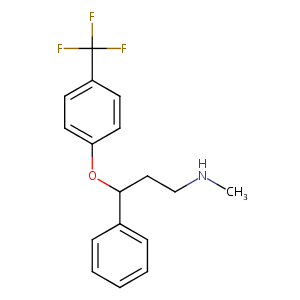 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
10 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
1 Approved Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
