| 1 |
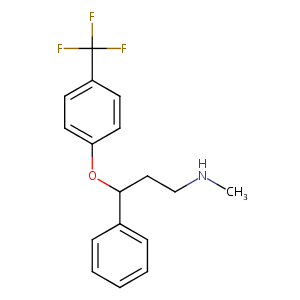
ClinicalTrials.gov (NCT01687478) A Study of Olanzapine and Fluoxetine for Treatment-resistant Depression
|
| 2 |
Fluoxetine FDA Label
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 203).
|
| 4 |
Fluoxetine to Reduce Intubation and Death After COVID19 Infection
|
| 5 |
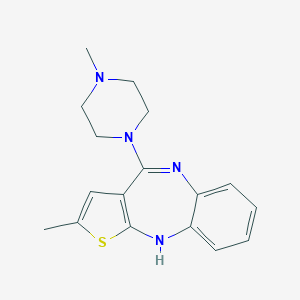
Olanzapine FDA Label
|
| 6 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 47).
|
| 7 |
A toxicogenomic approach to drug-induced phospholipidosis: analysis of its induction mechanism and establishment of a novel in vitro screening system. Toxicol Sci. 2005 Feb;83(2):282-92.
|
| 8 |
Transcriptome-based functional classifiers for direct immunotoxicity. Arch Toxicol. 2014 Mar;88(3):673-89.
|
| 9 |
Screening autism-associated environmental factors in differentiating human neural progenitors with fractional factorial design-based transcriptomics. Sci Rep. 2023 Jun 29;13(1):10519. doi: 10.1038/s41598-023-37488-0.
|
| 10 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services.
|
| 11 |
Fluoxetine and reversal of multidrug resistance. Cancer Lett. 2006 Jun 18;237(2):180-7.
|
| 12 |
O-Dealkylation of fluoxetine in relation to CYP2C19 gene dose and involvement of CYP3A4 in human liver microsomes. J Pharmacol Exp Ther. 2002 Jan;300(1):105-11.
|
| 13 |
Clinically significant psychotropic drug-drug interactions in the primary care setting. Curr Psychiatry Rep. 2012 Aug;14(4):376-90.
|
| 14 |
(R)-, (S)-, and racemic fluoxetine N-demethylation by human cytochrome P450 enzymes. Drug Metab Dispos. 2000 Oct;28(10):1187-91.
|
| 15 |
Antidepressant drugs in the elderly--role of the cytochrome P450 2D6. World J Biol Psychiatry. 2003 Apr;4(2):74-80.
|
| 16 |
Binding of CYP2C9 with diverse drugs and its implications for metabolic mechanism. Med Chem. 2009 May;5(3):263-70.
|
| 17 |
Molecular characterization of CYP2B6 substrates. Curr Drug Metab. 2008 Jun;9(5):363-73.
|
| 18 |
Effects of CYP2C19 genotype and CYP2C9 on fluoxetine N-demethylation in human liver microsomes. Acta Pharmacol Sin. 2001 Jan;22(1):85-90.
|
| 19 |
Age-dependent antidepressant pharmacogenomics: polymorphisms of the serotonin transporter and G protein beta3 subunit as predictors of response to fluoxetine and nortriptyline. Int J Neuropsychopharmacol. 2003 Dec;6(4):339-46. doi: 10.1017/S1461145703003663.
|
| 20 |
Mechanism-based inactivation of human cytochrome P4502C8 by drugs in vitro. J Pharmacol Exp Ther. 2004 Dec;311(3):996-1007.
|
| 21 |
Determination of phospholipidosis potential based on gene expression analysis in HepG2 cells. Toxicol Sci. 2007 Mar;96(1):101-14.
|
| 22 |
Inverse agonist and neutral antagonist actions of antidepressants at recombinant and native 5-hydroxytryptamine2C receptors: differential modulatio... Mol Pharmacol. 2008 Mar;73(3):748-57.
|
| 23 |
Effects of selective serotonin reuptake inhibitors on three sex steroids in two versions of the aromatase enzyme inhibition assay and in the H295R cell assay. Toxicol In Vitro. 2015 Oct;29(7):1729-35.
|
| 24 |
Antidepressant drugs activate SREBP and up-regulate cholesterol and fatty acid biosynthesis in human glial cells. Neurosci Lett. 2006 Mar 13;395(3):185-90. doi: 10.1016/j.neulet.2005.10.096. Epub 2005 Dec 1.
|
| 25 |
Fluoxetine inhibits the extracellular signal regulated kinase pathway and suppresses growth of cancer cells. Cancer Biol Ther. 2008 Oct;7(10):1685-93. doi: 10.4161/cbt.7.10.6664. Epub 2008 Oct 22.
|
| 26 |
Serum prolactin levels among outpatients with major depressive disorder during the acute phase of treatment with fluoxetine. J Clin Psychiatry. 2006 Jun;67(6):952-7. doi: 10.4088/jcp.v67n0612.
|
| 27 |
Anti-inflammatory properties of desipramine and fluoxetine. Respir Res. 2007 May 3;8(1):35. doi: 10.1186/1465-9921-8-35.
|
| 28 |
Acid sphingomyelinase-ceramide system mediates effects of antidepressant drugs. Nat Med. 2013 Jul;19(7):934-8. doi: 10.1038/nm.3214. Epub 2013 Jun 16.
|
| 29 |
Systems pharmacological analysis of drugs inducing stevens-johnson syndrome and toxic epidermal necrolysis. Chem Res Toxicol. 2015 May 18;28(5):927-34. doi: 10.1021/tx5005248. Epub 2015 Apr 3.
|
| 30 |
Fluoxetine mediates G0/G1 arrest by inducing functional inhibition of cyclin dependent kinase subunit (CKS)1. Biochem Pharmacol. 2008 May 15;75(10):1924-34. doi: 10.1016/j.bcp.2008.02.013. Epub 2008 Feb 17.
|
| 31 |
A cell-based Rb(+)-flux assay of the Kv1.3 potassium channel. Assay Drug Dev Technol. 2007 Jun;5(3):373-80. doi: 10.1089/adt.2006.004.
|
| 32 |
Downregulation of glucose-6-phosphatase expression contributes to fluoxetine-induced hepatic steatosis. J Appl Toxicol. 2021 Aug;41(8):1232-1240. doi: 10.1002/jat.4109. Epub 2020 Nov 12.
|
| 33 |
Palmitate increases the susceptibility of cells to drug-induced toxicity: an in vitro method to identify drugs with potential contraindications in patients with metabolic disease. Toxicol Sci. 2012 Oct;129(2):346-62. doi: 10.1093/toxsci/kfs208. Epub 2012 Jun 14.
|
| 34 |
Drug-induced long QT syndrome: hERG K+ channel block and disruption of protein trafficking by fluoxetine and norfluoxetine. Br J Pharmacol. 2006 Nov;149(5):481-9. doi: 10.1038/sj.bjp.0706892. Epub 2006 Sep 11.
|
| 35 |
The antidepressants maprotiline and fluoxetine induce Type II autophagic cell death in drug-resistant Burkitt's lymphoma. Int J Cancer. 2011 Apr 1;128(7):1712-23. doi: 10.1002/ijc.25477. Epub 2010 May 25.
|
| 36 |
Association of CYP1A1 and CYP1B1 inhibition in in vitro assays with drug-induced liver injury. J Toxicol Sci. 2021;46(4):167-176. doi: 10.2131/jts.46.167.
|
| 37 |
Establishment of ion channel and ABC transporter assays in 3D-cultured ReNcell VM on a 384-pillar plate for neurotoxicity potential. Toxicol In Vitro. 2022 Aug;82:105375. doi: 10.1016/j.tiv.2022.105375. Epub 2022 May 10.
|
| 38 |
Response to fluoxetine and serotonin 1A receptor (C-1019G) polymorphism in Taiwan Chinese major depressive disorder. Pharmacogenomics J. 2006 Jan-Feb;6(1):27-33. doi: 10.1038/sj.tpj.6500340.
|
| 39 |
Lymphoma cells protected from apoptosis by dysregulated bcl-2 continue to bind annexin V in response to B-cell receptor engagement: a cautionary tale. Leuk Res. 2006 Jan;30(1):77-80. doi: 10.1016/j.leukres.2005.05.018. Epub 2005 Aug 1.
|
| 40 |
Effect of repeated treatment with fluoxetine on tryptophan hydroxylase-2 gene expression in the rat brainstem. Pharmacol Biochem Behav. 2006 Sep;85(1):220-7. doi: 10.1016/j.pbb.2006.08.004. Epub 2006 Sep 18.
|
| 41 |
Association of brain-derived neurotrophic factor genetic Val66Met polymorphism with severity of depression, efficacy of fluoxetine and its side effects in Chinese major depressive patients. Neuropsychobiology. 2010;61(2):71-8. doi: 10.1159/000265132. Epub 2009 Dec 12.
|
| 42 |
Neuroleptic Drugs and PACAP Differentially Affect the mRNA Expression of Genes Encoding PAC1/VPAC Type Receptors. Neurochem Res. 2017 Apr;42(4):943-952. doi: 10.1007/s11064-016-2127-2. Epub 2016 Nov 30.
|
| 43 |
Olanzapine: an updated review of its use in the management of schizophrenia. Drugs. 2001;61(1):111-61.
|
| 44 |
Identification of P-glycoprotein substrates and inhibitors among psychoactive compounds--implications for pharmacokinetics of selected substrates. J Pharm Pharmacol. 2004 Aug;56(8):967-75.
|
| 45 |
Interactions between the cytochrome P450 system and the second-generation antipsychotics. J Psychiatry Neurosci. 2003 Mar;28(2):99-112.
|
| 46 |
[Olanzapine: pharmacology, pharmacokinetics and therapeutic drug monitoring]. Fortschr Neurol Psychiatr. 2001 Nov;69(11):510-7.
|
| 47 |
Evaluation of antipsychotic drugs as inhibitors of multidrug resistance transporter P-glycoprotein. Psychopharmacology (Berl). 2006 Sep;187(4):415-23. doi: 10.1007/s00213-006-0437-9. Epub 2006 Jun 30.
|
| 48 |
Dopamine receptor D2 gene is associated with weight gain in schizophrenic patients under long-term atypical antipsychotic treatment. Pharmacogenet Genomics. 2010 Jun;20(6):359-66. doi: 10.1097/FPC.0b013e3283397d06.
|
| 49 |
Drug-induced activation of SREBP-controlled lipogenic gene expression in CNS-related cell lines: marked differences between various antipsychotic drugs. BMC Neurosci. 2006 Oct 20;7:69.
|
| 50 |
Up-regulation of hepatic fatty acid transporters and inhibition/down-regulation of hepatic OCTN2 contribute to olanzapine-induced liver steatosis. Toxicol Lett. 2019 Nov;316:183-193. doi: 10.1016/j.toxlet.2019.08.013. Epub 2019 Aug 19.
|
| 51 |
In vitro approach to assess the potential for risk of idiosyncratic adverse reactions caused by candidate drugs. Chem Res Toxicol. 2012 Aug 20;25(8):1616-32. doi: 10.1021/tx300091x. Epub 2012 May 31.
|
| 52 |
Effects of olanzapine on serum protein phosphorylation patterns in patients with schizophrenia. Proteomics Clin Appl. 2015 Oct;9(9-10):907-16. doi: 10.1002/prca.201400148. Epub 2015 May 15.
|
| 53 |
The effects of olanzapine, risperidone, and haloperidol on plasma prolactin levels in patients with schizophrenia. Clin Ther. 2000 Sep;22(9):1085-96. doi: 10.1016/S0149-2918(00)80086-7.
|
| 54 |
Recovery from new-onset diabetes in a schizophrenic man after withdrawal of olanzapine. Psychosomatics. 2002 Jan-Feb;43(1):67-70. doi: 10.1176/appi.psy.43.1.67.
|
| 55 |
Hormonal markers of metabolic dysregulation in patients with severe mental disorders after olanzapine treatment under real-life conditions. J Clin Psychopharmacol. 2009 Apr;29(2):109-16. doi: 10.1097/JCP.0b013e31819b95fc.
|
| 56 |
Clozapine induces oxidative stress and proapoptotic gene expression in neutrophils of schizophrenic patients. J Clin Psychopharmacol. 2005 Oct;25(5):419-26. doi: 10.1097/01.jcp.0000177668.42640.fe.
|
| 57 |
No evidence for binding of clozapine, olanzapine and/or haloperidol to selected receptors involved in body weight regulation. Pharmacogenomics J. 2007 Aug;7(4):275-81. doi: 10.1038/sj.tpj.6500418. Epub 2006 Sep 19.
|
| 58 |
Effects of different antipsychotics and the antidepressant mirtazapine on glucose transporter mRNA levels in human blood cells. J Psychiatr Res. 2006 Jun;40(4):374-9. doi: 10.1016/j.jpsychires.2005.04.016. Epub 2005 Jul 5.
|
| 59 |
The differential effects of neuroleptic drugs and PACAP on the expression of BDNF mRNA and protein in a human glioblastoma cell line. Acta Neurobiol Exp (Wars). 2017;77(3):205-213.
|
| 60 |
Famotidine has a neuroprotective effect on MK-801 induced toxicity via the Akt/GSK-3/-catenin signaling pathway in the SH-SY5Y cell line. Chem Biol Interact. 2019 Dec 1;314:108823. doi: 10.1016/j.cbi.2019.108823. Epub 2019 Sep 26.
|
| 61 |
Effects of cyamemazine on hERG, INa, ICa, Ito, Isus and IK1 channel currents, and on the QTc interval in guinea pigs. Eur J Pharmacol. 2006 Feb 27;532(3):270-8. doi: 10.1016/j.ejphar.2005.12.079. Epub 2006 Feb 21.
|
| 62 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
| 63 |
Serotonin-1A receptor gene polymorphism and the ability of antipsychotic drugs to improve attention in schizophrenia. Adv Ther. 2010 May;27(5):307-13. doi: 10.1007/s12325-010-0035-4. Epub 2010 Jun 8.
|
| 64 |
Olanzapine-induced weight gain is associated with the -759C/T and -697G/C polymorphisms of the HTR2C gene. Pharmacogenomics J. 2009 Aug;9(4):234-41. doi: 10.1038/tpj.2009.18. Epub 2009 May 12.
|
| 65 |
DRD2/AKT1 interaction on D2 c-AMP independent signaling, attentional processing, and response to olanzapine treatment in schizophrenia. Proc Natl Acad Sci U S A. 2011 Jan 18;108(3):1158-63. doi: 10.1073/pnas.1013535108. Epub 2010 Dec 27.
|
| 66 |
Different impacts of aquaporin 4 and MAOA allele variation among olanzapine, risperidone, and paliperidone in schizophrenia. J Clin Psychopharmacol. 2012 Jun;32(3):394-7. doi: 10.1097/JCP.0b013e31825370f4.
|
| 67 |
MTHFR and risk of metabolic syndrome in patients with schizophrenia. Schizophr Res. 2010 Aug;121(1-3):193-8. doi: 10.1016/j.schres.2010.05.030. Epub 2010 Jun 12.
|
| 68 |
The SNAP-25 gene may be associated with clinical response and weight gain in antipsychotic treatment of schizophrenia. Neurosci Lett. 2005 May 6;379(2):81-9. doi: 10.1016/j.neulet.2004.12.037. Epub 2005 Jan 26.
|
| 69 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
|
|
|
|
|
|