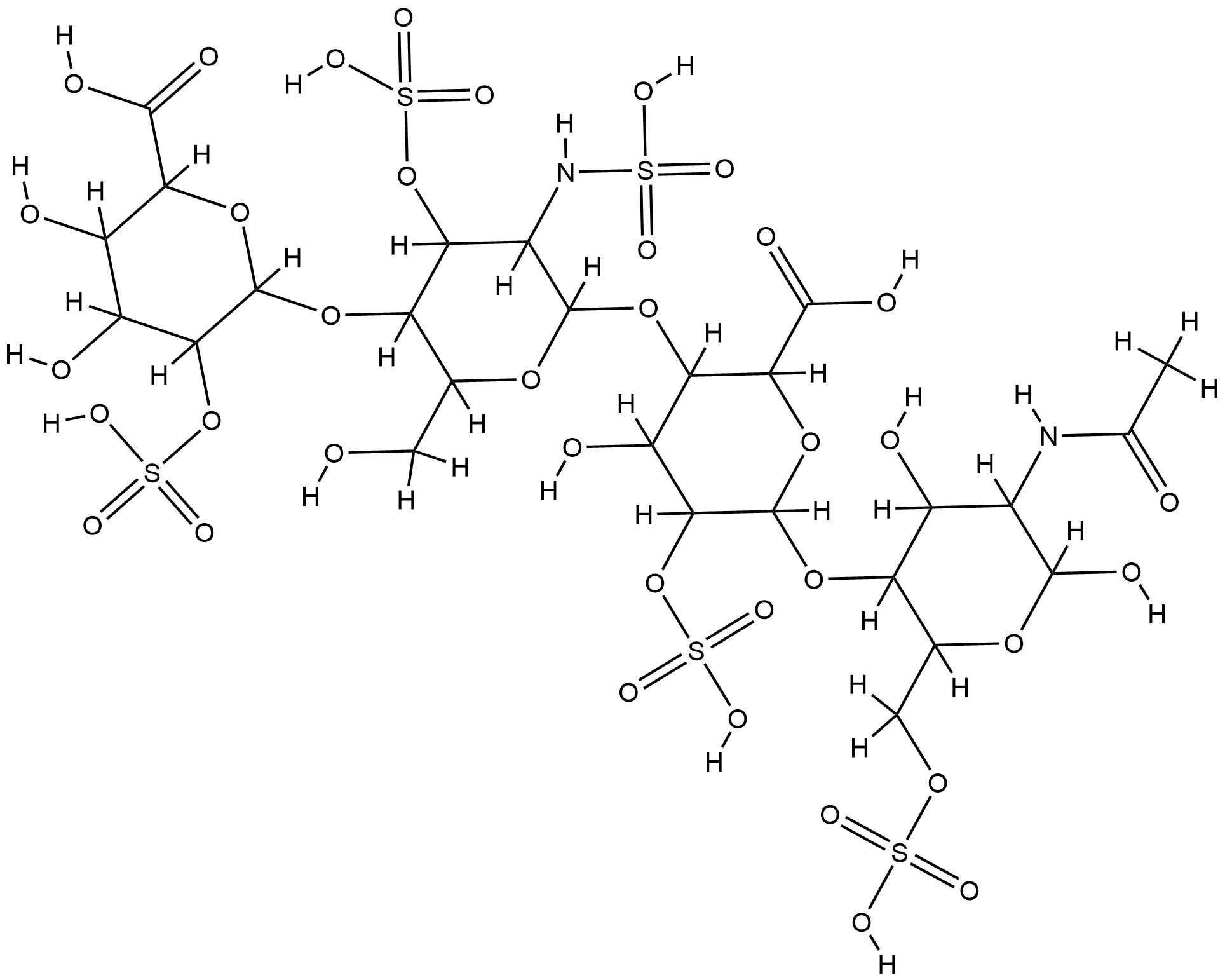
| 1 |
Heparin FDA Label
|
| 2 |
FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (ANDA) 090809.
|
| 3 |
Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020 Dec;9(1):727-732.
|
| 4 |
ClinicalTrials.gov (NCT03537586) A Single Center Diagnostic, Cross-sectional Study of Coronary Microvascular Dysfunction
|
| 5 |
ClinicalTrials.gov (NCT00433966) Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction
|
| 6 |
ClinicalTrials.gov (NCT00269880) A Study Comparing the Efficacy and Safety of Abciximab, an Anti-Platelet Therapy, in Combination With Two Different Heparin Regimens in Patients Undergoing Percutaneous Coronary Intervention.
|
| 7 |
ClinicalTrials.gov (NCT01965002) Feasibility of ExAblate MRI Guided High Intensity Focused Ultrasound Tx of Soft Tissue Tumors
|
| 8 |
ClinicalTrials.gov (NCT00885300) Cloxacillin as Prevention of Double Lumen Infection in Hemodialysis Patients
|
| 9 |
ClinicalTrials.gov (NCT02497326) Heparin for the Treatment of Burn Wound Pain
|
| 10 |
ClinicalTrials.gov (NCT03483207) Acute Mesenteric Venous Thrombosis.. in Assiut University Hospital Management Controversies
|
| 11 |
ClinicalTrials.gov (NCT05224388) Evaluating Dose Regimen of Intravenous Unfractionated Heparin and Low Molecular Weight Heparin in Critical Ill Patients Versus Critical Ill COVID-19 Patients Using Anti-Xa Levels.
|
| 12 |
ClinicalTrials.gov (NCT00836355) Enoxaparin and/or Minocycline in Acute Stroke
|
| 13 |
ClinicalTrials.gov (NCT01780987) AStudy To Evaluate Safety And Eficacy Of Apixaban In Japanese Acute Deep Vein Thrombosis (DVT) And Pulmonary Embolism (PE) Patients
|
| 14 |
ClinicalTrials.gov (NCT01464671) Angiomax or Unfractionated Heparin for Patients Undergoing Percutaneous Coronary Intervention
|
| 15 |
ClinicalTrials.gov (NCT04042324) A Study to Investigate the Effect of Triferic Plus Heparin Infusion Compared to Heparin Alone on Coagulation Parameters in Hemodialysis Patients
|
| 16 |
ClinicalTrials.gov (NCT01149304) Preventive Effect of Enoxaparin, Pentoxifylline and Ursodeoxycholic Acid to Radiation Induced Liver Toxicity
|
| 17 |
ClinicalTrials.gov (NCT04528888) Steroids and Unfractionated Heparin in Critically Ill Patients With Pneumonia From COVID-19 Infection
|
| 18 |
ClinicalTrials.gov (NCT04485429) Efficacy Assessment of Methylprednisolone and Heparin in Patients With COVID-19 Pneumonia
|
| 19 |
ClinicalTrials.gov (NCT02072434) Edoxaban vs. Warfarin in Subjects Undergoing Cardioversion of Nonvalvular Atrial Fibrillation (NVAF)
|
| 20 |
ClinicalTrials.gov (NCT04406389) Anticoagulation in Critically Ill Patients With COVID-19 (The IMPACT Trial)
|
| 21 |
ClinicalTrials.gov (NCT04409834) Prevention of Arteriovenous Thrombotic Events in Critically-Ill COVID-19 Patients Trial
|
| 22 |
ClinicalTrials.gov (NCT03664180) Comparison of Anticoagulation Prolongation vs. no Anticoagulation in STEMI Patients After Primary PCI
|
| 23 |
ClinicalTrials.gov (NCT03894904) Papaverine vs Heparin for Peripheral Arterial Catheter Patency in Pediatric Patients
|
| 24 |
ClinicalTrials.gov (NCT01917799) Aspirin, Heparin and Miscarriage
|
| 25 |
ClinicalTrials.gov (NCT02986594) Diagnosis and Treatment Strategy of Recurrent Spontaneous Abortion Associated With Thrombophilla
|
|
|
|
|
|
|