Details of the Drug Combinations
General Information of This Drug (ID: DM602WT)
| Drug Name | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Idelalisib; 870281-82-6; CAL-101; Zydelig; GS-1101; CAL101; CAL 101; (S)-2-(1-((9H-Purin-6-yl)amino)propyl)-5-fluoro-3-phenylquinazolin-4(3H)-one; UNII-YG57I8T5M0; CAL-101 (Idelalisib, GS-1101); YG57I8T5M0; CHEMBL2216870; CHEBI:82701; 5-Fluoro-3-phenyl-2-((S)-1-(9H-purin-6-ylamino)-propyl)-3H-quinazolin-4-one; AK145603; Idelalisib; CAL-101; (S)-2-(1-(9H-purin-6-ylamino)propyl)-5-fluoro-3-phenylquinazolin-4(3H)-one; 1146702-54-6; 5-FLUORO-3-PHENYL-2-[(1S)-1-(9H-PURIN-6-YLAMINO)PROPYL]-4(3H)-QUINAZOLINONE
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
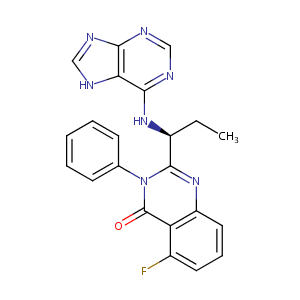 |
||||||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
6 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
9 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
