Details of the Drug Combinations
General Information of This Drug (ID: DMAIL4L)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Fludeoxyglucose F18; Fludeoxyglucose (18F); Fludeoxyglucose F 18; 105851-17-0; FLUDEOXYGLUCOSE F-18; 18FDG; UNII-SBT3GBX27W; SBT3GBX27W; CHEBI:31617; 2-(Fluoro-18F)-2-deoxy-alpha-glucopyranose; alpha-D-Glucopyranose, 2-deoxy-2-(fluoro-18F)-; [18F]FDG; 2-Deoxy-2-fluoro-(sup 18)F-alpha-D-glucopyranose; Flucis; alpha-D-Glucopyranose, 2-deoxy-2-(fluoro-(sup 18)F)-; F-18 FDG; Fluorodeoxyglucose F 18; AC1L9OYT; 2-Deoxy-2-(fluoro-18f)-a-D-glucopyranose; Fludeoxyglucose F 18 (TN); SCHEMBL135994; Fludeoxyglucose F 18 (USP)
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
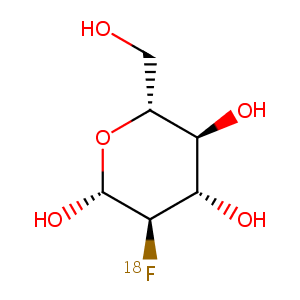 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
4 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||
References
