Details of the Drug Combinations
General Information of This Drug (ID: DMBOV5P)
| Drug Name | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Sodium oxybate; Oxybate sodium; Sodium Oxybutyrate; Anetamin; Sodium 4-hydroxybutyrate; 4-Hydroxybutyric acid sodium salt; Somsanit; 502-85-2; Catabate; Sodium gamma-hydroxybutyrate; Sodium oxybat; Gamma OH; 4-Hydroxybutyrate sodium; Natrium 4-hydroxybutyrat; Sodium Oxybate [USAN]; Sodium-4-hydroxybutyrate; gamma-Hydroxybutyrate sodium; LUMRYZ; gamma-Hydroxy sodium butyrate; Butanoic acid, 4-hydroxy-, monosodium salt; 4-Hydroxybuttersaeure natriumsalz; gamma-Hydroxybutyrate sodium salt; sodium 4-hydroxybutanoate; WY-3478; Oxybate (sodium); EINECS 207-953-3; JZP-6; NSC 84223; gamma-Hydroxybutyric acid, sodium salt; Butanoic acid, 4-hydroxy-, sodium salt; DTXSID3048940; EB 27; Hydroxybutyric acid monosodium salt; 4-Hydroxybutyric acid monosodium salt; NSC-84223; Sodium gammahydroxybutyrate; UNII-7G33012534; WY 3478; Butyric acid, 4-hydroxy-, monosodium salt; DTXCID6028866; GHB.sodium salt; BUTYRIC ACID, 4-HYDROXY-, SODIUM SALT; .gamma.-hydroxybutyrate sodium salt; XYWAV COMPONENT SODIUM OXYBATE; 7G33012534; SODIUM OXYBATE COMPONENT OF XYWAV; Sodium oxybate (USAN); SODIUM OXYBATE (MART.); SODIUM OXYBATE [MART.]; Oxybate, Sodium; Oxybutyrate, Sodium; NSC84223; GHB Sodium Salt (Sodium Gammahydroxybutyrate); 4 Hydroxybutyrate Sodium; NCGC00247714-01; Sodium gamma Hydroxybutyrate; Acetamide,2,2-dichloro-N-[(1R,2R)-2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl]-,rel-; Xyrem (TN); SCHEMBL61823; SODIUM OXYBATE [HSDB]; SODIUM OXYBATE [VANDF]; DEA No. 2012; CHEMBL1200682; OXYBATE SODIUM [WHO-DD]; SODIUM OXYBATE [EMA EPAR]; XYGBKMMCQDZQOZ-UHFFFAOYSA-M; HMS2091E15; ; A-Hydroxybutyric acid sodium salt; HY-B1187; Tox21_112871; gamma-Hydroxybutyric acid sodium salt; SODIUM OXYBATE [ORANGE BOOK]; Gamma Hydroxybutyric Acid preparations; GHB.sodium salt, 1mg/ml in Methanol; AKOS006221428; CCG-212465; CS-4796; DB09072; CAS-502-85-2; FT-0626615; D05866; H-4040; SODIUM SALT OF GAMMA-HYDROXYBUTYRIC ACID; .GAMMA.-HYDROXYBUTYRATE SODIUM SALT [MI]; Butanoic acid, 4-hydroxy-, sodium salt (1:1); Q7553347; ?-Hydroxybutyric Acid Sodium Salt (1.0 mg/mL in Methanol)
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||||||
| Structure |
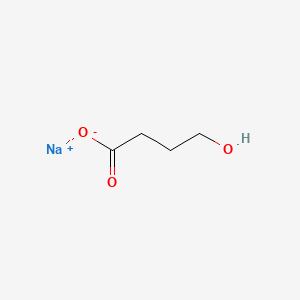 |
||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
