Details of the Drug Combinations
General Information of This Drug (ID: DMDEXQ0)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Adenuric; TEI; Uloric; Febuxostat [USAN]; S1547; TMX 67; Tei 6720; TMX-67; Tei-6720; Uloric (TN); Febuxostat (JAN/USAN/INN); TMX-67, Adenuric, Uloric, Febuxostat; 111GE013; 2-(3-CYANO-4-ISOBUTOXY-PHENYL)-4-METHYL-5-THIAZOLE-CARBOXYLIC ACID; 2-(3-Cyano-4-(2-methylpropoxy)phenyl)-4-methylthiazole-5-carboxylic acid;2-(3-Cyano-4-isobutoxyphenyl)-4-methyl-5-thiazolecarboxylic acid; 2-[3-cyano-4-(2-methylpropoxy)phenyl]-4-methyl-1,3-thiazole-5-carboxylic acid
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
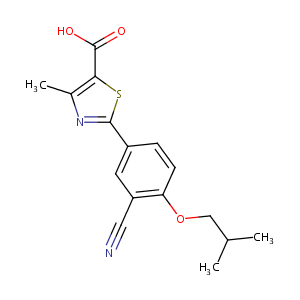 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
12 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
